October 1, 2019
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
Chloraserrtone A
Chloranthus serratus (Chloranthaceae) can be found throughout southern China. It has been used as a traditional Chinese medicine. The characteristic secondary metabolites of the genus Chloranthus are sesquiterpenoids, especially the sesquiterpenoid dimers. These exhibit a wide range of bioactivity. About 70 lindenane-type sesquiterpenoid dimers have been isolated from this genus over the past three decades.
Chloranthus, and most of the sesquiterpenoid dimers are biosynthesized from two lindenane-type sesquiterpenoid monomers connected via a six-membered ring (C-4−C-15−C-9′−C-8′−C-6−C-5) by an endo Diels−Alder cycloaddition reaction.
Compounds with new skeletons are constantly being isolated from this genus, and the structural diversity of sesquiterpenoid dimers has attracted the attention of an increasing number of researchers.
Chloraserrtone A (1), a new sesquiterpenoid dimer with two lindenane-type sesquiterpenoid monomers bridged by two six-membered rings, was isolated from Chloranthus serratus by Bai et al [1]. A combination of UV, IR, NMR, HRESIMS, and X-ray diffraction data were used to elucidate the structure of 1. Compound 1 represents the first lindenane-type sesquiterpenoid dimer with an extremely unique skeleton.
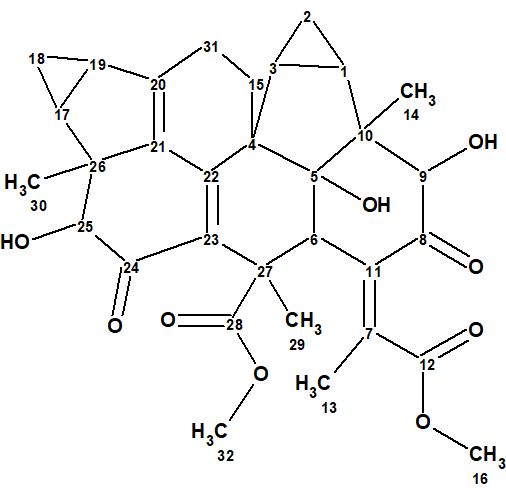
1
Chloraserrtone A was obtained as colorless crystals with a specific rotation of [α]D 20 −39 (c 0.2, CH2Cl2) [UV (MeOH) λmax (log ε) 266 (3.55), 204 (3.73) nm]. The molecular formula of 1 was established as C32H36O9 by the HRESIMS ion at m/z 587.2243 [M + Na]+ (calcd 587.2252), implying 15 indices of hydrogen deficiency. IR absorption bands at 3352, 1730, and 1636 cm−1 suggested the presence of hydroxy, carbonyl, and olefinic groups.
The molecular formula of 1 and the NMR spectroscopic data tabulated in [1] were entered into ACD/Structure Elucidator (Table 1).
Table 1. NMR spectroscopic data of Chloraserrtone A.
| Label | δC | δC сalc | CHn | δH | COSY | H to C HMBC |
| C 1 | 26.600 | 26.430 | CH | 1.800 | 0.47, 1.46 | C 3, C 4, C 9, C 5 |
| C 2 | 8.200 | 9.240 | CH2 | 0.470 | 1.46, 1.80 | C 3, C 1, C 4, C 10 |
| C 2 | 8.200 | 9.240 | CH2 | 0.640 | ||
| C 3 | 25.300 | 25.770 | CH | 1.460 | 0.47, 1.80 | C 5, C 22 |
| C 4 | 52.000 | 52.450 | C | |||
| C 5 | 84.400 | 85.340 | C | |||
| C 6 | 50.800 | 50.280 | CH | 3.270 | C 29, C 27, C 10, C 5, C 7, C 11, C 28, C 8 |
|
| C 7 | 127.700 | 136.420 | C | |||
| C 8 | 198.900 | 199.100 | C | |||
| C 9 | 77.400 | 85.350 | CH | 4.520 | C 14, C 1, C 10, C 5, C 8 | |
| C 10 | 54.300 | 51.170 | C | |||
| C 11 | 145.800 | 142.560 | C | |||
| C 12 | 171.200 | 170.640 | C | |||
| C 13 | 17.500 | 18.720 | CH3 | 1.860 | C 7, C 11, C 12, C 8 | |
| C 14 | 15.400 | 17.560 | CH3 | 0.840 | C 1, C 10, C 9, C 5 | |
| C 15 | 33.600 | 27.370 | CH2 | 2.270 | 2.42 | C 31, C 3, C 4, C 5, C 20, C 22 |
| C 15 | 33.600 | 27.370 | CH2 | 1.850 | ||
| C 16 | 52.800 | 52.800 | CH3 | 3.850 | C 12 | |
| C 17 | 26.500 | 29.810 | CH | 2.100 | 0.44, 1.90 | C 25, C 21, C 20 |
| C 18 | 14.400 | 14.270 | CH2 | 1.030 | C 19, C 17, C 26, C 20 | |
| C 18 | 14.400 | 14.270 | CH2 | 0.440 | 1.90, 2.10 | |
| C 19 | 26.200 | 25.830 | CH | 1.900 | 0.44, 2.10 | C 31, C 26, C 21 |
| C 20 | 150.000 | 154.290 | C | |||
| C 21 | 136.900 | 139.840 | C | |||
| C 22 | 153.500 | 152.030 | C | |||
| C 23 | 125.400 | 133.470 | C | |||
| C 24 | 199.400 | 198.150 | C | |||
| C 25 | 81.100 | 80.420 | CH | 4.170 | C 30, C 17, C 26, C 24 | |
| C 26 | 54.700 | 53.950 | C | |||
| C 27 | 48.100 | 50.470 | C | |||
| C 28 | 176.500 | 175.270 | C | |||
| C 29 | 23.400 | 21.030 | CH3 | 1.430 | C 27, C 6, C 23, C 28 | |
| C 30 | 15.800 | 18.890 | CH3 | 1.020 | C 17, C 26, C 25, C 21 | |
| C 31 | 25.300 | 21.930 | CH2 | 2.420 | 2.27 | C 19, C 15, C 4, C 21, C 20 |
| C 31 | 25.300 | 21.930 | CH2 | 2.670 | C 15, C 21, C 20 | |
| C 32 | 52.900 | 52.800 | CH3 | 3.600 | C 28 |
The automatically generated Molecular Connectivity Diagram (MCD), which displays all atoms along with their properties and HMBC and COSY connectivities, is shown in Figure 1.
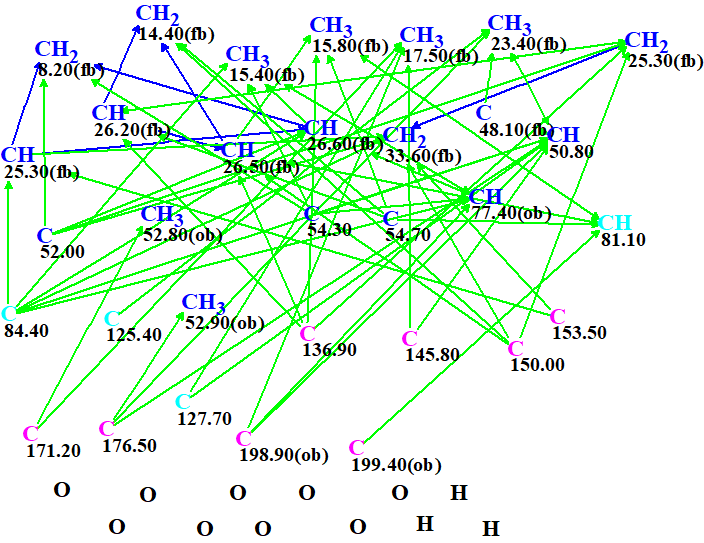
MCD overview. Atom properties (hybridization and possibility to be connected to a heteroatom) were set by the program automatically based on the system knowledge base. Four carbon atoms, (C 81.1, C 84.4, C 125.4 and C 127.7) colored in light blue, are assigned ambiguous hybridization “not sp” (sp2 or sp3). No manual edits were made on the MCD.
After checking the MCD for the presence of contradictions the program informed us that “The minimum number of non-standard connectivities (NSCs) is 1”, and the carbon atom CH3 17.50 was indicated as a potential origin of a NSC. Therefore Fuzzy Structure Generation (FSG) was enabled with the options automatically determined by the program. Results: k = 77,606 (spectral and structural filtering) → 10 (removal of duplicates) → 6, tg = 2 m 25 s.
13C and 1H NMR chemical shift prediction was carried out for the structural output file using three empirical methods common for ADC/SE (HOSE code based, neural networks, incremental approach), and the structures were ranked in increasing order of average deviation dA(13C) between experimental and calculated chemical shifts. The three top ranked structures are presented in Figure 2.
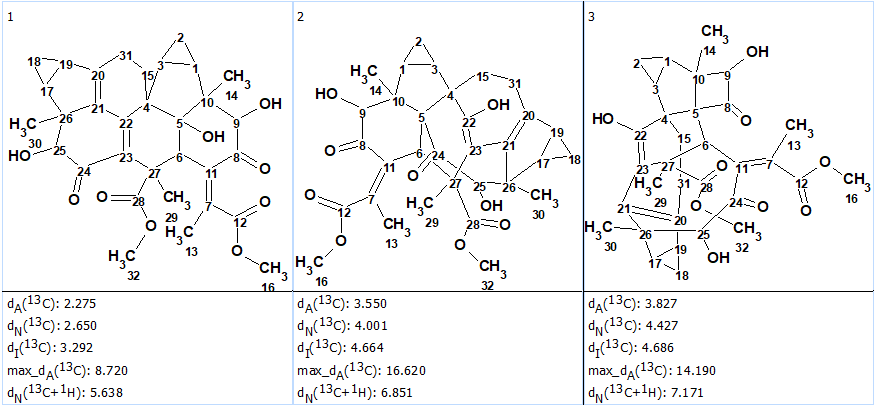
We see that all three methods of NMR chemical shift prediction pointed to structure #1 as the best one. Comparison of this structure with structure of chloraserrtone A determined by authors [1] and confirmed by X ray analysis shows that they are identical.
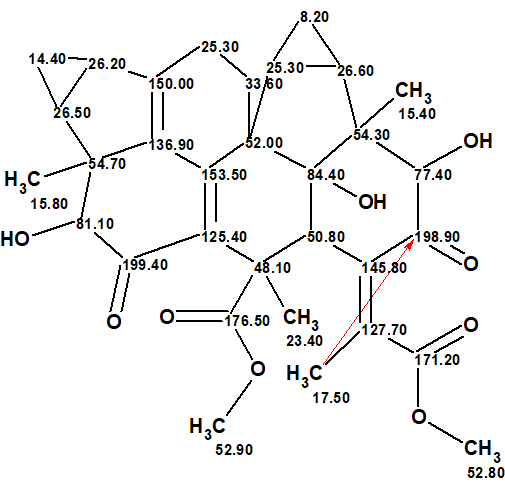
Thus, the structure of an unusual new natural product whose molecule contains 8 fused rings was elucidated by ACD/Structure Elucidator fully automatically in 2.5 min. The elucidated structure together with the 13C chemical shifts which were assigned by the program is shown below. A red arrow directed from CH3 17.50 to C 198.90 denotes a long-range HMBC correlations of four chemical bonds length. This is the atom that was selected by the program as the potential origin of the NSC, after the analysis of the MCD.
References
- B. Bai, S.-X. Ye, D.-P. Yang, L.-P. Zhu, G.-H. Tang, Y.-Y. Chen, G. Q. Li, Z.-M. Zhao. (2019). Chloraserrtone A, a Sesquiterpenoid Dimer from Chloranthus serratus. J. Nat. Prod., 82: 407−411.


