
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

The following names are retained:Examples to Rule C-317.1
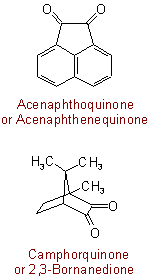
317.2 - Radicals formed by loss of hydrogen from a quinone are named by changing the ending "-quinone" to "-quinonyl", the quinone grouping having priority over the point of attachment for lowest locants.
See Recommendations'93 R-5.6.2Example to Rule C-317.2


Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact [email protected]