
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

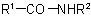 and
and 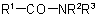 (also analogous sulfonamides, etc.) are named by one of the following methods:
(also analogous sulfonamides, etc.) are named by one of the following methods:
824.1 - The compound is named as an N-substituted amide, the substituents 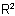 and
and 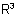 being cited as prefixes.
being cited as prefixes.
Note: This method is particularly suitable when the group 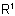 in the acyl residue R1CO- is more complex than the group
in the acyl residue R1CO- is more complex than the group 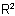 , or
, or 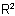 and
and
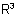 , in the amine residue
, in the amine residue 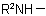 or
or 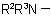 .
.
824.2 - Alternatively, the acyl group is named as an N-substituent of the base.Examples to Rule C-824.1
Note: This method is particularly suitable when the group 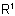 in the acyl residue
in the acyl residue 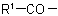 is less complex than the group
is less complex than the group 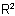 , or
, or 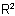 and
and
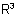 , in the amine residue
, in the amine residue 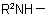 or
or 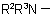 , and for use with derivatives of nitrogen containing heterocyclic compounds.
, and for use with derivatives of nitrogen containing heterocyclic compounds.
824.3 - As a further alternative, for mono-N-substituted primary amidesExamples to Rule C-824.2
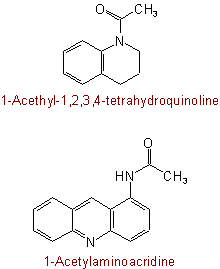
(but see also Rule C-824.3)
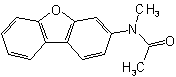

(but see also Rule C-824.4)
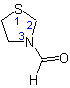
3-Formylthiazolidine
(see Rule C-304.3)
or 3-Thiazolidinecarbaldehyde
(see Rule C-304.1)
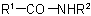 the group
the group 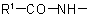 is treated as a substituent into the compound
is treated as a substituent into the compound
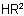 and is named by changing the ending "-amide" to "amido-" or "-carboxamide" to "carboxamido-".
and is named by changing the ending "-amide" to "amido-" or "-carboxamide" to "carboxamido-".
824.4 - For di-N-substituted primary amidesExamples to Rule C-824.3
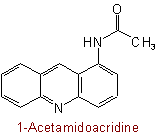
(but see also Rule C-824.2)
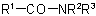 the procedure of Rule C-824.3 is applied, the less complex of the groups
the procedure of Rule C-824.3 is applied, the less complex of the groups 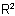 and
and
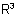 being named as substituent of the amido or carboxamido group with locant N.
being named as substituent of the amido or carboxamido group with locant N.
Note: The procedures of Rules C-824.3 and C-824.4 are particularly suitable when the groupExample to Rule C-824.4
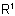 in the acyl residue
in the acyl residue 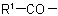 is less complex than
the group
is less complex than
the group 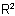 , or
, or 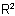 and
and 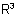 , in the amine residue
, in the amine residue 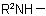 or
or 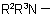 ; they are not applicable to derivatives of N-acyl
nitrogen-containing heterocyclic systems.
; they are not applicable to derivatives of N-acyl
nitrogen-containing heterocyclic systems.
See Recommendations'93 R-5.7.8


Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact webmaster@acdlabs.com