
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

 , and R.S. Cahn, C.K. Ingold, and V. Prelog, Angew. Chem.,
78, 413-447 (1966); and V. Prelog and G. Helmchen, Angew. Chem., Int. Ed., 21, 567-583 (1982)) and are preceded when necessary by appropriate locants. For carbon
(or other atoms) to which four different ligands are attached in the system Cabcd may be depicted as in 1, where a>b>c>d
, and R.S. Cahn, C.K. Ingold, and V. Prelog, Angew. Chem.,
78, 413-447 (1966); and V. Prelog and G. Helmchen, Angew. Chem., Int. Ed., 21, 567-583 (1982)) and are preceded when necessary by appropriate locants. For carbon
(or other atoms) to which four different ligands are attached in the system Cabcd may be depicted as in 1, where a>b>c>d .
.
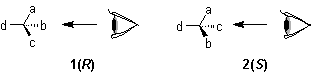
If the model 1 is viewed from the side remote from the fourth member d, the path from a to b to c traces a clockwise direction, and the system has the 'R' configuration; if passing from a to b to c traces an anticlockwise direction, as in 2, the configuration is 'S'.
The sequence rule itself is the method by which the groups a, b, c, d are assigned priorities. It contains five sub-rules, but only the first two are mentioned here, the other sub-rules being needed in relatively few cases.
Sub-rule 1: Higher atomic number precedes lower.
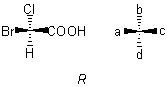
Here, the atomic numbers of the atoms Br, Cl, C, H attached to the central carbon atom are arranged in that order by sub-rule 1, giving the model shown, which is in the R configuration.
When two or more atoms attached to the chiral centre are alike, the comparison is extended through each of these atoms to succeeding atoms until a sequence priority can be established on the same
basis. Multiple bonds are considered as two or three single bonds to the same atom. Thus, a 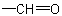 group is treated as
group is treated as
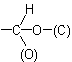 and
and 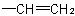 as
as 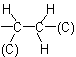
where (O) and (C) are 'duplicate representations' of the respective atoms which, if needed for further comparison, are attached to 'phantom atoms' having neither atomic number nor mass. Since O is
preferred to C when the sequences O, (O), H and C, (C), H are compared, the 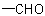 group has sequence rule priority over the
group has sequence rule priority over the 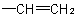 .
.
Similarly, aExamples to R-7.2.1
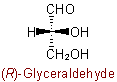
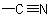 group is treated as
group is treated as 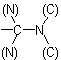
Sub-rule 2: Higher mass number precedes lower (applies only when sub-rule 1 does not decide priority).
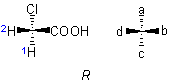
In this case, the mass numbers allow the atoms to be arranged in the order Cl, C, 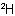 ,
, 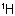 , resulting in an R configuration.
, resulting in an R configuration.
If a molecule contains more than one chiral centre, this procedure is applied to each, and the configuration is expressed as a set or R,S symbols. In names of compounds, the symbols R and S, with locants if needed, are written in parentheses, followed by a hyphen, in front of the complete name or appropriate substituent.
Examples to R-7.2.1



Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact webmaster@acdlabs.com