
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

R-8.3.1.1 The structural formula of a specifically labelled compound is written in the usual way, but with the appropriate nuclide symbol(s)
and multiplying subscript, if any, enclosed in square brackets. Other principles used in writing the formula are described in Section H of the 1979 edition of the IUPAC Nomenclature
of Organic Chemistry , page 514.
, page 514.
Note: Although the formula for a specifically labelled compound does not represent the composition of the bulk material, which usually consists overwhelmingly of the isotopically unmodified compound, it does indicate the presence of the compound of chief interest, the isotopically substituted compound.Examples to R-8.3.1.1
Isotopically
substituted
compoundwhen
added toIsotopically
unmodified
compoundgives
rise toSpecifically
labelled
compoundCH4 CH4
A specifically labelled compound is (a) singly labelled when the isotopically substituted compound has only one isotopically modified atom, for example 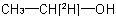 ; (b)
multiply labelled when the isotopically substituted compound has more than one modified atom of the same element at the same position or at different position, for example
; (b)
multiply labelled when the isotopically substituted compound has more than one modified atom of the same element at the same position or at different position, for example
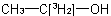 and
and 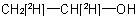 ; or (c) mixed labelled when the isotopically substituted compound has more than one kind of modified atom, for example
; or (c) mixed labelled when the isotopically substituted compound has more than one kind of modified atom, for example 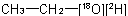 .
.
R-8.3.1.2 The name of a specifically labelled compound is formed by inserting in square brackets nuclide symbol(s) preceded by any necessary locants before the name or preferable before the denomination for that part of the compound that is isotopically modified. When polylabelling is possible, the number of atoms that have been labelled is always specified as a subscript to the atomic symbol(s) even in case of monolabelling. This is necessary in order to distinguish between a specifically and a selectively or nonselectively labelled compound.
Examples to R-8.3.1.2



Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact [email protected]