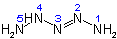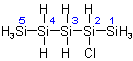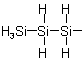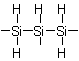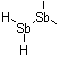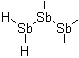Bring the power of IUPAC naming to your desktop!
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.
Chains and Rings with Regular Patterns of Heteroatoms
Rule D-4.1 Homogeneous Chains
4.11 - A compound having an unbranched chain of identical heteroatoms saturated with hydrogen atoms to the extent required by the standard bonding
numbers given in Rule D-1.61 is named by citing successively:
(a) the appropriate multiplying prefix: "di", "tri", "tetra", etc. The terminal vowel is not elided even if the "a" term begins with the same vowel,
(b) the appropriate "a" term given in
Table D-I denoting the heteroatom; except for chains of sulfur, selenium and tellurium atoms for which the "a" terms are "sulfa", "sela", and "tella",
(c) the termination
"ne".
If the bonding number is that indicated in Rule D-1.61 as normal bonding number, then the appropriate "a" term is Used unmodified. If, however, the bonding number of the
heteroatom differs from the normal bonding number or is not given in Rule D-1.61, then the "a" term is always modified by affixing a 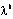 symbol, according to Rule
D-1.62.
symbol, according to Rule
D-1.62.
Any valence not involved in a chain-linkage will be considered as satisfied by a hydrogen atom.
Examples to Rule D-4.11
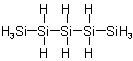
Pentasilane
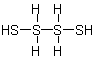
Tetrasulfane
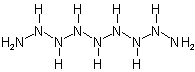
Nonaazane
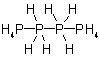

4.12 - The name of a compound having an unbranched chain of identical heteroatoms with fewer hydrogen atoms than required to satisfy the standard bonding numbers given in Rule
D-1.61 is derived from the name of the corresponding saturated chain by changing the ending "-ane" to "-ene" or "-yne" and adding any necessary locants and multipliers.
Example to Rule D-4.12
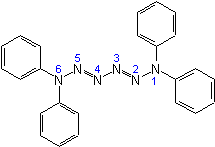
1,1,6,6-Tetraphenyl-2,4-hexaazadiene
4.13 - A compound having an unbranched chain of identical heteroatoms with associated hydrogen atoms is numbered by Arabic numerals from one terminal heteroatom to the other. That heteroatom
is numbered 1 as will give the lowest locants to:
to:
(a) unsaturation, according to Rule A-3.3,
(b) substituents.
These priorities will be considered in sequence until a unique numbering is obtained.
Examples to Rule D-4.13
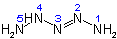
2-Pentaazene
(Priority: 2 before 3)
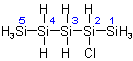
2-Chloropentasilane
(Priority: 2 before 4)
4.14 - A radical consisting of an unbranched chain of identical heteroatoms is named by adding the endings "-yl", "-diyl", "-triyl", etc. to the name of the corresponding heteroatom chain,
coined according to Rules D-4.11 and D-4.12, eliding the final "e" of the name of the heteroatom chain before "y". The name is preceded by the locant(s) of the heteroatom(s)
carrying the free valence. 
The chain of the radical is numbered from one end to the other so that the lowest possible locants are given to:
are given to:
(a) free valence(s),
(b) unsaturation, according to Rule A-3.3,
(c) substituents.
The abbreviated forms silyl, germyl, stannyl and plumbyl are used instead of silanyl, etc., to distinguish between disilanyl, i.e. two silyl groups (2SiH3), and disilanyl, i.e. one disilanyl group
(Si2H5), etc.
Examples to Rule D-4.14
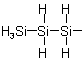
1-Trisilanyl
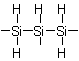
1.2.3-Trisilanetriyl
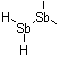
1,1-Distibanediyl
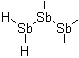
1,1,2-Tristibanetriyl


This HTML reproduction of Sections A, B and C of IUPAC "Blue Book" is as close as possible to the
published version [see Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, Oxford, 1979.
Copyright 1979 IUPAC.] If you need to cite these rules please quote this reference as their source.
Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact [email protected]
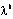 symbol, according to Rule
D-1.62.
symbol, according to Rule
D-1.62.
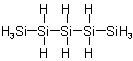
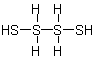

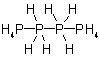


 to:
to: