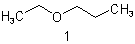
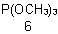
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.
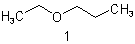 |
 |
 |
 |
 |
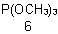 |
 |
 |
 |
|||||
| Structure | Parent structure (Class Name) |
Operation | Name | Reference | |
 |
|||||
| 1 | Propane(ether) | substitutive functional class  |
1-Ethoxypropane Ethyl propyl ether |
R-1.2.1 R-1.2.3.3.2 |
|
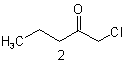
| 2 | Pentane(ketone) | substitutive functional class  |
1-Chloropentan-2-one Chloromethyl propyl ketone |
R-1.2.1 R-1.2.3.3.2 |
|
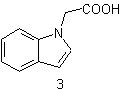
| 3 | Acetic acid Indole and Acetic acid |
 |
substitutive conjunctive |
1H-Indol-1-ylacetic acid 1H-Indole-1-acetic acid |
R-1.2.1 R-1.2.4.1 |
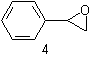
| 4 | Styrene (oxide) Oxirane |
additive substitutive |
Styrene oxide 2-Phenyloxirane |
R-1.2.3.3 R-1.2.1 |
|
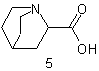
| 5 | Quinuclidine Bicyclooctane |
 |
substitutive replacement substitutive |
Quinuclidine-2-carboxylic acid 1-Azabicyclo[2.2.2] octane-2- carboxylic acid |
R-1.2.1 R-1.2.2.1 R-1.2.1 |
| 6 | (phosphite) Phosphane |
functional class substitutive coordination (additive) |
Trimethyl phosphite Trimethoxyphosphane Trimethoxophosphorus |
R-1.2.3.3.2 R-1.2.1 R-1.2.3.1 |
|
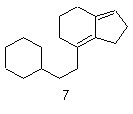
| 7 | Gonane Indene |
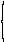 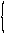 |
ring cleavage substractive substitutive additive |
9,10-Secogona-8(14),13(17)- diene 7-(2-Cyclohexylethyl)-2,4,5,6- tetrahydro-1H-indene |
R-1.2.6.2 R-1.2.5.2 R-1.2.1 R-1.2.3.1 |
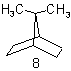
| 8 | Bornane Bicycloheptane |
subtractive substitutive |
10-Norbornane 7,7-Dimethylbicyclo- [2.2.1]heptane |
R-1.2.5.1 R-1.2.1 |
|


Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact webmaster@acdlabs.com