
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

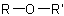 ,
, 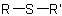 ,
, 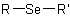 and
and 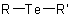 are called generically "ethers", "sulfides", "selenides", and
"tellurides", respectively, and are named by one of three method: substitutive, functional class, or replacement.
are called generically "ethers", "sulfides", "selenides", and
"tellurides", respectively, and are named by one of three method: substitutive, functional class, or replacement.
R-5.5.4.1 Substitutive names are formed by prefixing the name of the group 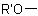 ,
, 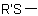 ,
, 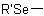 , or
, or
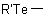 to the name of the parent hydride corresponding to R
to the name of the parent hydride corresponding to R .
.
R-5.5.4.2 Functional class names are formed by citing the names of the groups R andExamples to R-5.5.4.1
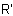 in alphabetical order followed by the class name "ether", "sulfide",
"selenide", or "telluride", each as a separate word.
in alphabetical order followed by the class name "ether", "sulfide",
"selenide", or "telluride", each as a separate word.
R-5.5.4.3 Replacement nomenclature (see R-1.2.2) is used to name linear polyethers, polysulfides, etc. It is especially advantageous for unsymmetrical structures with several chalcogen atoms and those with several different chalcogen atoms.Examples to R-5.5.4.2
R-5.5.4.4 Cyclic ethers. An oxygen atom directly attached to two atoms that are already part of a ring system, or to two carbon atoms of a chain, may be named (a) as a heterocycle, following the appropriate recommendations for naming heterocycles, including the use of the prefix "epoxy" as a bridge prefix, in which case it is a nondetachable prefix (see R-0.1.8); or (b) by using the prefix "epoxy" substitutively, in which case it is detachable (see R-0.1.8) and therefore alphabetized along with any other substitutive prefixes. The latter method is particularly useful when it is desired to preserve the name of a specific structure, for example, in naming natural products such as steroids and carotenoids.Examples to R-5.5.4.3
Note: The class name "oxide" has also been used additively to name cyclic ethers of the second kind, for example, ethylene oxide and styrene oxide.
Examples to R-5.5.4.4


Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact [email protected]