
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

1.2 - Heterocyclic systems whose unsaturation is less than the one corresponding to the maximum number of non-cumulative double bonds are named by using the prefixes "dihydro-", "tetrahydro-", etc.Examples to Rule B-1.1
In the case of 4- and 5-membered rings, a special termination is used for the structures containing one double bond, when there can be more than one non-cumulative double bond.
 |
||
| No. of members of the partly saturated rings |
Rings containing nitrogen |
Rings containing no nitrogen |
 |
||
| 4 | -etine | -etene |
| 5 | -oline | -olene |
1.3 - Multiplicity of the same hetero atom is indicated by a prefix "di-", "tri-", etc., placed before the appropriate "a" term (Table B-I).Examples to Rule B-1.2

1.4 - If two or more kinds of "a" terms occur in the same name, their order of citation is in order of their appearance in Table B-I of Rule B-1.1.Example to Rule B-1.3

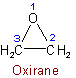
1.51 - The position of a single hetero atom determines the numbering in a monocyclic compound.Examples to Rule B-1.4
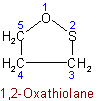
1.52 - When the same hetero atom occurs more than once in a ring, the numbering is chosen to give the lowest locants to the hetero atoms.Example to Rule B-1.51
1.53 - When hetero atoms of different kinds are present, the locant 1 is given to a hetero atom which is as high as possible in Table B-I. The numbering is then chosen to give the lowest locants to the hetero atoms.Example to Rule B-1.52
See Recommendations'93 R-2.3.3Examples to Rule B-1.53
The numbering must begin with the sulfur atom. This condition eliminates 2,1,4-thiadiazine. Then the nitrogen atoms receive the lowest possible locant, which eliminates 1,3,6-thiadiazine.
The numbering has to begin with the sulfur atom. The choice of this atom is determined by the set of locants which can be attributed to the remaining hetero atoms of any kind.
As the set 1,2,5 is lower than 1,3,4 or 1,5,4 in the usual sense, the name is 1,5,2-dithiazine.



Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact [email protected]