
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

(a) A nitrogen-containing component.
(b) A component containing a heteroatom (in the absence of nitrogen) as high as possible in Table B-I.Example to Rule B-3.1(a)
Benz[z]isoquinoline
not Pyrido[2,3-b]naphthalene
(c) A component containing the greatest number of rings.Example to Rule B-3.1(b)
Thieno[2,3-b]furan
not Furo[2,3-b]thiophene
(d) A component containing the largest possible individual ring.Example to Rule B-3.1(c)
7H-Pyrazino[2,3-c]carbazole
not 7H-Indolo[3,2-f]quinoxaline
(e) A component containing the greatest number of hetero atoms of any kind.Example to Rule B-3.1(d)
2H-Furo[3,2-b]pyran
not 2H-pyrano[3,2-b]furan
(f) A component containing the greatest variety of hetero atoms.Example to Rule B-3.1(e)
5H-Pyrido[2,3-d]-o-oxazine
not o-Oxazino[4,5-b]pyridine
(g) A component containing the greatest number of hetero atoms first listed in Table B-I.Examples to Rule B-3.1(f)
1H-Pyrazolo[4,3-d]oxazole
not 1H-Oxazolo[5,4-c]pyrazole
4H-Imidazo[4,5-d]thiazole
not 4H-Thiazolo[4,5-d]imidazole
(h) If there is a choice between components of the same size containing the same number and kind of hetero atoms choose as the base component that one with the lower numbers for the hetero atoms before fusion.Example to Rule B-3.1(g)
Selenazolo[5,4-f]benzothiazole
not Thiazolo[5,4-f]benzoselenazole
3.2 - If a position of fusion is occupied by a hetero atom, the names of the component rings to be fused are so chosen as both to contain the hetero atom.Example to Rule B-3.1(h)
Pyrazino[2,3-d]pyridazine
3.3 - The following contracted fusion prefixes may be used: furo, imidazo, isoquino, pyrido, pyrimido, quino and thieno.Example to Rule B-3.2
Imidazo[2,1-b]thiazole
3.4 - In peripheral numbering of the complete fused systems, the ring system is oriented and numbered according to the principles of Rule A-22. When there is a choice of orientations, it is made in the following sequence in order to:Examples to Rule B-3.3
Furo[3,4-c]cinnoline
4H-Pyrido[2,3-c]carbazole
(a) Give low numbers to hetero atoms;
 Benzo[b]furan |
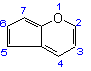 Cyclopenta[b]pyran |
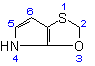 4H-[1,3]Oxathiolo[5,4-b]pyrrole  |
|
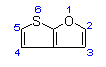
Thieno[2,3-b]furan
(c) Allow carbon atoms common to two or more rings to follow the lowest possible numbers (see Rules A-22.2 and A-22.3). [A hetero atom common to two rings is numbered according to Rule B-3.4(e)], thus:
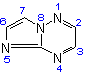 Imidazo[1,2-b][1,2,4]triazine  |
|||
| not | 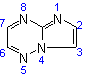 |
or | 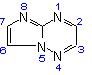 |
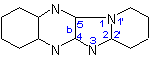 |
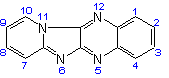
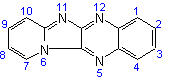
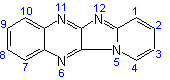
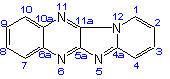 Pyrido[1',2':1,2]imidazo[4,5-b]-quinoxaline |
| not |
 |
| or |
 |
| or |
 |
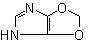
4H-1,3-Dioxolo-[4,5-d]imidazole
(e) The ring is numbered as for hydrocarbons but numbers are given to all hetero atoms even when common to two or more rings. Interior hetero atoms are numbered last following the shortest path from the highest previous number.
3.5 - As exceptions, two-ring systems in which a benzene ring is fused to a hetero ring may be named by prefixing numbers indicating the positions of the hetero atoms to benzo followed by the name of the heterocyclic component. Numbering is assigned by the principles set forth in Rule B-3.4 (a), (b), and (d)The names provided by this rule may also be used for components of more complex fused systems.
See Recommendations'93 R-2.4.1Examples to Rule B-3.5
3-Benzoxepin
not Benz[d]oxepin
4H-3,1-Benzoxazine
not 4H-Benz[d][1,3]oxazine
1H-Pyrrolo[1,2-b][2]benzazepine
not 1H-Benzo[e]pyrrolo[1,2-a]azepine



Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact [email protected]