
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

 in the following
order:
in the following
order:
(a) from the nature of the compound, determine the type(s) of nomenclature operations (see Section R-1.2) to be used. Although the so-called "substitutive nomenclature" is emphasized in these recommendations, other kinds of names, for example, functional class names, are often given, usually as alternatives;
(b) determine the kind of characteristic group to be cited as suffix (if any) or as a functional class name. Only one kind of characteristic group (known as the principal group) can be cited as
suffix or functional class name  . All substituents not so cited must be specified as prefixes;
. All substituents not so cited must be specified as prefixes;
(c) determine the parent hydride, including any appropriate nondetachable prefixes [detailed rules for choice of the principal chain, the preferred ring or ring system,
the functional parent compound, or conjunctive components are described in the 1979 edition of the IUPAC
Nomenclature of Organic Chemistry (see, for example, Rule C-12];
(see, for example, Rule C-12];
(d) name the parent hydride and the principal characteristic group, if any, or the functional parent compound;
(e) determine infixes and/or prefixes [with the appropriate multiplying prefixes (see Table 11)],
and number the structure as far as possible ;
;
(f) name the detachable substitutive prefixes and complete the numbering of the structure, if necessary;
(g) assemble the components into a complete name, using alphabetical order for all substitutive prefixes.
In substitutive nomenclature, some characteristic groups can be denoted either as prefixes or suffixes (see Table 5), but others only as prefixes (see Table 9). Functional class names differ in that a separate word (or suffix in some languages) designating the name of a functional class is associated with a "radical" name designating the remainder of the structure.
The characteristic groups that can be cited as suffixes in substitutive nomenclature are not necessarily identical with the groups designated by the name of a corresponding functional class when
functional class names are formed (e.g., butanone and ethyl methyl ketone, where -one denotes 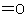 and ketone denotes
and ketone denotes 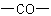 ).
).
The characteristic groups listed in Table 9 are always cited as prefixes to the name of the parent structure (see Sections R-2 and R-3). Multiplying prefixes and locants are added as necessary.
Characteristic groups other than those given in Table 9 may be cited as either suffixes, if available, or prefixes to the name of the parent hydride.Example to R-4.1
1,2-Dichlorocyclohexane
(the complete prefix is 1,2-dichloro-)
If characteristic groups other than those given in Table 9 are present, one (and only one) kind may be cited as suffix (the principal characteristic group) .
.
When a compound contains more than one kind of characteristic group not given in Table 9, the principal characteristic group is the one that characterizes the class occurring earliest (i.e., nearest to the top) in Table 10. All other characteristic groups are cited as prefixes. Some suffixes and prefixes to be used with the general classes listed in Table 10 are given in Table 5.
If, and only if, the complete suffix (that is, the suffix itself plus its multiplying affix, if any) begins with a vowel, a terminal "e" (if any) of the preceding parent name is elided (see Section R-0). Elision or retention of the terminal "e" is independent of the presence of numerals between it and the following letter (see Section R-0).
The basic numerical terms (multiplying affixes) given in Table 11 are used by direct joining without hyphen.
Numerical terms for use as multiplying prefixes for complex entities, such as substituted substituents, are obtained by adding the ending "-kis" to the numerical term defined as above. However, the ending "-kis" is not used with "mono-". As exceptions, "bis-" is used for the number 2 and "tris-" for 3.
The following multiplying prefixes are used in names for unbranched assemblies of three or more identical repeating units.Examples to R-4.1
4 tetrakis-231 hentriacontadictakis-
| 2 | bi- | 7 | septi- |
| 3 | ter- | 8 | octi- |
| 4 | quater- | 9 | novi- |
| 5 | quinque- | 10 | deci- |
| 6 | sexi- |
(a) that no characteristic group is expressed as a suffix (instead, a suffix such as "-yl" or "-ylidene", etc., is used); and
(b) that the point of attachment of the complex substituent has the lowest permissible locant.
When the parent hydride (principal chain, ring system), principal group and other substituents have been selected and named, the numbering of the complete compound is allocated.
Insofar as the preceding rules leave a choice, the starting point and direction of numbering of a compound are chosen so as to give lowest locants to the following structural features (if present) considered successively in the order listed until a decision is reached.
(a) fixed numbering (naphthalene, etc.)
(b) heteroatoms in heterocycles
(c) indicated hydrogen (see R-1.3)
(d) principal group named as suffix
(e) heteroatoms in an acyclic parent structure
(g) substituents named as prefixes.
The various components having been selected, named and numbered, any necessary additive and subtractive modifications are made, and the complete name is assembled, prefixes being arranged in alphabetical order.




Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact webmaster@acdlabs.com