
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

R-5.1.4.2 Heterogeneous silicon hydrides: siloxanes and analogues. Silicon compounds having the general formulaExamples to R-5.1.4.1
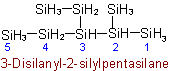
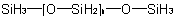 are called "siloxanes" generically and are
named on the basis of parent hydride names such as "disiloxane", "trisiloxane", etc., according to the number of silicon atoms (n+2) in the chain. Sulfur, selenium, tellurium, and saturated
nitrogen analogues are named in the same way as "silathianes", "silaselenanes", "silatelluranes" and "silazanes", respectively (see also R-2.2.3.2).
are called "siloxanes" generically and are
named on the basis of parent hydride names such as "disiloxane", "trisiloxane", etc., according to the number of silicon atoms (n+2) in the chain. Sulfur, selenium, tellurium, and saturated
nitrogen analogues are named in the same way as "silathianes", "silaselenanes", "silatelluranes" and "silazanes", respectively (see also R-2.2.3.2).
Monocyclic siloxanes having the general formulaExamples to R-5.1.4.2
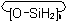 are named "cyclotrisiloxane", "cyclotetrasiloxane", etc., according to the number of silicon atoms (n) present. Sulfur,
selenium, tellurium, and saturated nitrogen analogues are named as "cyclosilathianes", "cyclosilaselenanes", etc., respectively (see also R-2.3.3.2).
are named "cyclotrisiloxane", "cyclotetrasiloxane", etc., according to the number of silicon atoms (n) present. Sulfur,
selenium, tellurium, and saturated nitrogen analogues are named as "cyclosilathianes", "cyclosilaselenanes", etc., respectively (see also R-2.3.3.2).
Bi- and polycyclic siloxanes, silathianes, silaselenanes, silatelluranes, and silazanes are named by citing a prefix defining the ring structure, such as "bicyclo[3.3.1]" and "spiro[5.7]", followed by a numerical term giving the number of silicon atoms present in the ring system, the whole being attached to "siloxane", "silathiane", "silaselenane", "silatellurane", "silazane", as appropriate. In the case of bi- and polycyclic silazanes, the prefix 1Si- or 1N- is used when necessary to indicate the atom at the bridgehead that is numbered 1 (see also R-2.4.2). Additional details for naming these compounds are given in the 1979 edition of the IUPAC Nomenclature of Organic ChemistryExample to R-5.1.4.2
 (see Rule D-6).
(see Rule D-6).
Examples to R-5.1.4.2



Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact [email protected]