
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

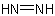 is named "diazene"
is named "diazene" ,
,  and the groups derived from it, i.e.,
and the groups derived from it, i.e., 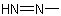 and
and 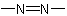 , are named systematically as diazenyl and diazenediyl, respectively
, are named systematically as diazenyl and diazenediyl, respectively (see R-3.1.4).
(see R-3.1.4).
R-5.3.3.1 Azo compounds. Compounds with the general structure 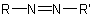 , where R and
, where R and 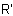 may be alike or different, are known generically as
"azo compounds". However, these compounds may be more systematically named substitutively as derivatives of the parent hydride diazene
may be alike or different, are known generically as
"azo compounds". However, these compounds may be more systematically named substitutively as derivatives of the parent hydride diazene .
.
A monoazo compound with the general structureExamples to R-5.3.3.1
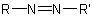 in which R is substituted by a principal characteristic group is named on the basis of the parent hydride, RH, substituted by an
organyldiazenyl group,
in which R is substituted by a principal characteristic group is named on the basis of the parent hydride, RH, substituted by an
organyldiazenyl group, 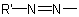 . If both R and
. If both R and 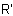 are substituted by the same number of the principal characteristic group, a multiplicative name (see R-0.2.3.3.10)
may be used.
are substituted by the same number of the principal characteristic group, a multiplicative name (see R-0.2.3.3.10)
may be used.
Bisazo compounds and more complex analogues are named on the basis of the parent structure "diazene", in the absence of a more preferred parent compound.Examples to R-5.3.3.1
R-5.3.3.2 Azoxy compounds. N-Oxides of azo compounds having the general structureExamples to R-5.3.3.1
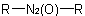 or
or 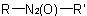 are known generically as "azoxy
compounds" and are named by adding the separate word "oxide" to the name of the corresponding azo compound (see R-5.3.3.1)
are known generically as "azoxy
compounds" and are named by adding the separate word "oxide" to the name of the corresponding azo compound (see R-5.3.3.1) . In an unsymmetrical
compound, the position of the azoxy oxygen atom is expressed by the locant 1 or 2
. In an unsymmetrical
compound, the position of the azoxy oxygen atom is expressed by the locant 1 or 2 .
.
An azoxy compound in which the general structure isExamples to R-5.3.3.2
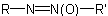 or
or 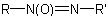 , in which R is substituted by a principal characteristic group, is named on the basis of the parent hydride,
RH, substituted by the
, in which R is substituted by a principal characteristic group, is named on the basis of the parent hydride,
RH, substituted by the 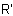 -azoxy group in which the position of the oxygen atom is denoted by the prefix NNO-, ONN-, or NON-, as appropriate.
-azoxy group in which the position of the oxygen atom is denoted by the prefix NNO-, ONN-, or NON-, as appropriate.
R-5.3.3.3 Diazonium compounds. Compounds with the general structureExample to R-5.3.3.2
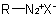 are named by adding the suffix "-diazonium" to the name of the parent hydride RH
followed by the name of the ion
are named by adding the suffix "-diazonium" to the name of the parent hydride RH
followed by the name of the ion 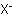 as a separate word.
as a separate word.
R-5.3.3.4 Azo compounds with the general structure R-N=N-X should be named as derivatives of the parent structure diazene, HN=NH.Examples to R-5.3.3.3
R-5.3.3.5 Diazo compounds. Compounds containing a group N2 attached by one nitrogen atom to one carbon atom are named by adding a prefix "diazo-" to the name of the parent hydride.Examples to R-5.3.3.4
Examples to R-5.3.3.5


Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact [email protected]