 -methylquinoline-2-pentanoic acid
-methylquinoline-2-pentanoic acid 
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.
 -methylquinoline-2-pentanoic acid
-methylquinoline-2-pentanoic acid 
(a) The suffix '-oic acid' denotes the presence of a carboxy 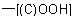 group attached to the parent hydride 'pentane' (see R-5.7.1), giving the structure and numbering shown as (I), below.
Note that the carbon atom of the carboxy group is included in the parent chain and that the numbering of the chain, although shown, is not that used to indicate substituents of the chain in the name
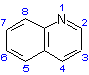
of the complete compound. The structure and numbering of the heterocyclic component, quinoline, is given as (II).
group attached to the parent hydride 'pentane' (see R-5.7.1), giving the structure and numbering shown as (I), below.
Note that the carbon atom of the carboxy group is included in the parent chain and that the numbering of the chain, although shown, is not that used to indicate substituents of the chain in the name
of the complete compound. The structure and numbering of the heterocyclic component, quinoline, is given as (II).
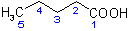 (I)
(I)
 (II)
(II)
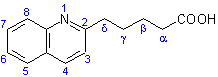
The locant '2' is the position on the heterocyclic component, quinoline, at which the acyclic component, pentanoic acid, is attached. No locant is given to denote the position of the acyclic component attached to the heterocycle, because, by definition, the acyclic component must be attached at the atom furthest from the principal group (see R-1.2.4.1). The structure and numbering of the conjunctive parent structure, quinoline-2-pentanoic acid is shown as (III).
 (III)
(III)
 ', respectively, indicate the presence of these groups as substituents at positions '3' and '
', respectively, indicate the presence of these groups as substituents at positions '3' and ' ' of the conjunctive parent
structure.
' of the conjunctive parent
structure.
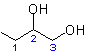 (IV)
(IV)
(c) Attaching structure (IV) and the methyl group to the conjunctive parent structure (III) completes the structure of the compound shown below.
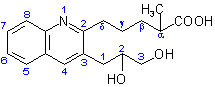




Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact webmaster@acdlabs.com