
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

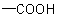 , that conceptually
replace CH3 groups terminating an acyclic hydrocarbon chain are denoted by adding the suffix "-oic acid" or "-dioic acid" (see Table 12) to the name of the acyclic hydrocarbon with elision
of its final "e" before "o".
, that conceptually
replace CH3 groups terminating an acyclic hydrocarbon chain are denoted by adding the suffix "-oic acid" or "-dioic acid" (see Table 12) to the name of the acyclic hydrocarbon with elision
of its final "e" before "o".
If an unbranched chain is directly linked to more than two carboxy groups, these carboxy groups are named from the parent hydride by substitutive use of a suffix such as "-tricarboxylic acid", etc.Examples to R-5.7.1.1
Other carboxylic acids are named by adding the suffix "-carboxylic acid" to the name of a parent hydride.Example to R-5.7.1.1
When another group is present that has priority for citation as a suffix (see Table 10, R-4.1. and Table 5, R-3.2.1.1) or when all carboxylic acid groups cannot be described in the suffix, a carboxylic acid group is indicated by the prefix "carboxy-".Examples to R-5.7.1.1
The name of a monovalent or divalent acyl group formed by removal of theExamples to R-5.7.1.1
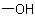 group from each carboxy group of a carboxylic acid denoted by an "-oic acid" suffix or having a trivial name
(see Table 28) is derived from the name of the corresponding acid by changing the ending "-oic acid" or "-ic acid" to "-oyl" or "-yl", respectively.
group from each carboxy group of a carboxylic acid denoted by an "-oic acid" suffix or having a trivial name
(see Table 28) is derived from the name of the corresponding acid by changing the ending "-oic acid" or "-ic acid" to "-oyl" or "-yl", respectively.
An acyl group derived from an acid named by means of the suffix "-carboxylic acid" is named by changing the suffix to "-carbonyl".Examples to R-5.7.1.1
Some trivial names are retained (see R-9.1, Table 28(a)).Examples to R-5.7.1.1
R-5.7.1.2 Substituted carboxylic acids
R-5.7.1.2.1 Hydroxy, alkoxy, and oxo acids. Some trivial names for hydroxy and alkoxy acids are retained (see Section R-9.1, Table 28(b)). The names of
carboxylic acids containing an aldehydic group attached to, or a ketonic group contained in the principal chain or parent ring system, are generally derived from the names of the corresponding simple
carboxylic acids by adding prefixes such as "oxo-", "dioxo-", etc., denoting 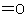 substituents, or "formyl-", demoting a
substituents, or "formyl-", demoting a 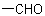 substituent.
substituent.
When a dicarboxylic acid has a trivial name (see R-9.1, Table 28(a)), the replacement of one of the carboxy groups by an aldehydic group may be denoted by changing the ending "-ic acid" into "-aldehydic acid" (see Table 12(b)).Examples to R-5.7.1.2.1
R-5.7.1.2.2 Amic and anilic acids. When a dicarboxylic acid has a retained trivial name (see R-9.1, Table 28(a)) and when one of its carboxy groups is replaced by a carboxamide groupExamples to R-5.7.1.2.1
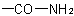 , the resulting amic acid is named by replacing the suffix "-ic acid" of the name of the dicarboxylic acid by the suffix "-amic acid". Some trivial names are
retained (see R-9.1, Table 28(c)).
, the resulting amic acid is named by replacing the suffix "-ic acid" of the name of the dicarboxylic acid by the suffix "-amic acid". Some trivial names are
retained (see R-9.1, Table 28(c)).
N-Phenyl derivatives of amic acids may be named by changing the "-amic acid" suffix to "-anilic acid". Positions on the phenyl ring are indicated by primed numbers. The nitrogen atom is indicated by "N".Examples to R-5.7.1.2.2
R-5.7.1.2.3 Amino acids. Retained trivial names for amino carboxylic acids are given in R-9.1, Table 28(b) and Table 28(c).Example to R-5.7.1.2.2
 -Amino carboxylic acids
are treated in specialized rules
-Amino carboxylic acids
are treated in specialized rules .
.
R-5.7.1.3 Modification of cardoxylic acid suffixes
R-5.7.1.3.1 Peroxy acids. Acids containing the group 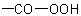 are called generically "peroxy acids" and are named by placing prefixes such as "peroxy-", "monoperoxy-", and
"diperoxy-", as appropriate, before a trivial (see R-9.1, Table 28(a)) or systematic name of an "-oic acid", or before a "carboxulic acid" or "-dicarboxylic acid" suffix (see
Table 13). When another group is present that has priority for citation as a suffix (see Table 10, R-4.1 and Table 5, R-3.2.1.1), a peroxy
carboxylic acid group is indicated by the prefix "hydroperoxycarbonyl-".
are called generically "peroxy acids" and are named by placing prefixes such as "peroxy-", "monoperoxy-", and
"diperoxy-", as appropriate, before a trivial (see R-9.1, Table 28(a)) or systematic name of an "-oic acid", or before a "carboxulic acid" or "-dicarboxylic acid" suffix (see
Table 13). When another group is present that has priority for citation as a suffix (see Table 10, R-4.1 and Table 5, R-3.2.1.1), a peroxy
carboxylic acid group is indicated by the prefix "hydroperoxycarbonyl-".
R-5.7.1.3.2 Imidic, hydrazonic, and hydroximic acids. The name of an acid in which the carbonyl oxygen atom of a carboxylic acid group has been replaced by aExamples to R-5.7.1.3.1
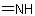 ,
,
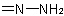 , or
, or 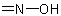 group is formed by modifying the "-oic" or "-carboxylic" suffix of a systematic name of an acid, or the "-ic acid" ending of the trivial name of an acid to
"-imidic" or "-carboximidic acid", "-ohydrazonic" or "-carbohydrazonic acid", "-ohydroximic" or "-carbohydroximic acid", respectively (see Table 13 and R-3.4).
group is formed by modifying the "-oic" or "-carboxylic" suffix of a systematic name of an acid, or the "-ic acid" ending of the trivial name of an acid to
"-imidic" or "-carboximidic acid", "-ohydrazonic" or "-carbohydrazonic acid", "-ohydroximic" or "-carbohydroximic acid", respectively (see Table 13 and R-3.4).
R-5.7.1.3.3 Hydroxamic acids. The name of an acid in which the hydrixy group of the carboxy group has been replaced by aExamples to R-5.7.1.3.2
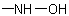 group can be formed by modifying the "-oic
acid" or "-carboxylic acid" suffix of a systematic name of an acid, or the "-ic acid" ending of a trivial acid name to "-ohydroxamic acid" or "-carbohydroxamic acid" (see Table 13); however,
in these recommendations, hydroxamic acids are preferably named as N-hydroxy amides.
group can be formed by modifying the "-oic
acid" or "-carboxylic acid" suffix of a systematic name of an acid, or the "-ic acid" ending of a trivial acid name to "-ohydroxamic acid" or "-carbohydroxamic acid" (see Table 13); however,
in these recommendations, hydroxamic acids are preferably named as N-hydroxy amides.
R-5.7.1.3.4 Thiocarboxylic and thiocarbonic acids. Replacement of oxygen atom(s) of a carboxylic acid group or of carbonic acid by another chalcogen is indicated by the affixes "thio", "seleno", and "telluro". These names do not differentiate between tautomeric forms of mixed chalcocarboxylic or chalcocarbonic acids; such nonspecificity may be shown in a formula by a structure such as:Example to R-5.7.1.3.3
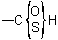
In names, tautomeric groups in mixed chalcocarboxylic and chalcocarbonic acids, such as 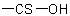 and
and 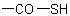 , may be distinguished by prefixing italic element symbols, such as
O- or S-, respectively, to the term "acid" (see Table 13); or by prefixes such as "hydroxy(thiocarbonyl)-" and "sulfanylcarbonyl-".
, may be distinguished by prefixing italic element symbols, such as
O- or S-, respectively, to the term "acid" (see Table 13); or by prefixes such as "hydroxy(thiocarbonyl)-" and "sulfanylcarbonyl-".
Replacement of oxygen by (an)other chalcogen atom(s) in a carboxylic acid having a retained trivial name or in carbonic acid is indicated by prefixes, such as "thio-", "seleno-", "dithio-", etc.
Replacement of oxygen by (an)other chalcogen atom(s) in a carboxylic acid having a systematic name is indicated by modifying the "-oic acid" or "-carboxylic acid" suffix to suffixes such as "-thioic acid", "-selenoic acid", "-carbodithioic acid"; and "-carboselenothioic acid", and the prefix "carboxy-" to prefixes such as "thiocarboxy-", "diselenocarboxy-", and "selenothiocarboxy-".Examples to R-5.7.1.3.4
Examples to R-5.7.1.3.4



Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact webmaster@acdlabs.com