
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

Neutral salts of oragnic acids are named by citing the cation(s) followed by the name of the anion as a separate word. Diverse cations are cited in alphabetical order.
Acid salts of polybasic organic acids are named in the same way as the neutral salts, the remaining acid hydrogen atom(s) being indicated by the word "hydrogen" (or "dihydrogen", etc., as appropriate) inserted between the name(s) of the cation(s) and the name of the anion from which it is separated by spacesExamples to R-5.7.4.1
 . Ionic substituents such as
. Ionic substituents such as
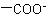 ,
, 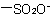 , and
, and 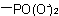 are described by prefix names such as "carboxylato-", "sulfonato-", and "phosphonato-", respectively.
are described by prefix names such as "carboxylato-", "sulfonato-", and "phosphonato-", respectively.
Sodium hydrogen 3-[3-(carboxylatomethyl)-2-naphthyl]propanoateExamples to R-5.7.4.1
R-5.7.4.2 Esters. Fully esterified acids are named in the same way as neutral salts except that names of alkyl or aryl, etc., groups, cited in alphabetical order when more than one, replace the names of the cations.
Structural specificity for esters of thio- or selenocarboxylic acids is provided by the appropriate italic element symbol, such as S- or O-, prefixed to the name of the alkyl, aryl, etc., group, as needed.Examples to R-5.7.4.2
Functional class nomenclature is often used for naming esters of natural products and in index nomenclature.Examples to R-5.7.4.2
Partial (acid) esters of polybasic acids and their salts are named by the procedures for neutral esters and acid salts; the components present are cited in the order: cation, alkyl or aryl group, hydrogen, anion. Numerical locants and italic element symbols are added as necessary to provide specificity.Examples to R-5.7.4.2
When, in an ester with the general structureExamples to R-5.7.4.2
(note that the numbering of the diacid is retained)
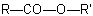 , another group is present that has priority for citation as a suffix (see Table 10, R-4.1, and Table 5,
R-3.2.1.1), or when all ester groups cannot be described by the above methods, an ester group is indicated by prefixes such as "alkoxycarbonyl-" or "aryloxycarbonyl-" for the group
, another group is present that has priority for citation as a suffix (see Table 10, R-4.1, and Table 5,
R-3.2.1.1), or when all ester groups cannot be described by the above methods, an ester group is indicated by prefixes such as "alkoxycarbonyl-" or "aryloxycarbonyl-" for the group
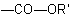 , or "acyloxy-" for the group
, or "acyloxy-" for the group 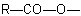 .
.
Functional class nomenclature can also be used, particularly in indexes, to describe an ester in the presence of a group with priority for citation as a principal characteristic group.Examples to R-5.7.4.2
Example to R-5.7.4.2


Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact webmaster@acdlabs.com