
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

R-5.7.5.1 Lactones. Inrtamolecular esters of hydroxy carboxylic acids are "lactones" and are named as heterocycles or by substituting "-olactone"
for the "-ic acid" ending of a trivial name of a hydroxy acid, or "-lactone" for the "-ic acid" ending of a systematic "-oic acid" name for the nonhydroxylated parent acid, and inserting a locant
designating the position of the hydroxy group between the "o" and "lactone"
Heterocycles in which one or more (but not all) rings of a polycyclic ring system are lactones are named bu adding the suffix "-carbolactone" (denoting a cyclicExample to R-5.7.5.1
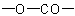 group) to the name
of the ring system left after the
group) to the name
of the ring system left after the 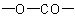 residue is replaced by two hydrogen atoms, preceded by a pair of locants indicating the points of attachment of the carbonyl group and the oxygen
atom of the lactone, respectively; the locant for the carbonyl group is cited first, and, if there is a choice, is the lower locant. Multiplying prefixes and pairs of locants separated by a colon
denote the precence of two or more lactone rings.
residue is replaced by two hydrogen atoms, preceded by a pair of locants indicating the points of attachment of the carbonyl group and the oxygen
atom of the lactone, respectively; the locant for the carbonyl group is cited first, and, if there is a choice, is the lower locant. Multiplying prefixes and pairs of locants separated by a colon
denote the precence of two or more lactone rings.
R-5.7.5.2 Sultones. Intramolecular esters of hydroxy sulfonic acids are called "sultones" and are named as heterocycles or by citing the term "sultone" denoting the cyclicExamples to R-5.7.5.1
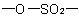 group after the name of the appropriate parent hydride preceded by a pair of locants describing the points of attachment of the sulfonyl group and oxygen atom, respectively; the
locant for the sulfonyl group is cited first and, if there is a choice, is the lower locant. Multiplying prefixes and pairs of locants separated by a colon are used to indicate two or more sultone
rings.
group after the name of the appropriate parent hydride preceded by a pair of locants describing the points of attachment of the sulfonyl group and oxygen atom, respectively; the
locant for the sulfonyl group is cited first and, if there is a choice, is the lower locant. Multiplying prefixes and pairs of locants separated by a colon are used to indicate two or more sultone
rings.
R-5.7.5.3 Lactams and lactims. Nitrogen analogues of lactones having the groupExamples to R-5.7.5.2
 as part of a ring or ring system are called generically "lactams" and
their tautomers,
as part of a ring or ring system are called generically "lactams" and
their tautomers, 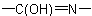 , are "lactims". These compounds are named as heterocyclic compounds or in accordance with R-5.7.5.1 using "-lactam" or "-lactim", respectively, in place
of "-lactone".
, are "lactims". These compounds are named as heterocyclic compounds or in accordance with R-5.7.5.1 using "-lactam" or "-lactim", respectively, in place
of "-lactone".
Names such as "propiolactam" and "butyrolactim" are not included in these recommendations.
R-5.7.5.4 Sultams. Nitrogen analogues of sultones having the groupExamples to R-5.7.5.3
Tetrahydropyrrol-2-one
Pyrrolidin-2-one (2-Pyrrolidone
)
Butano-4-lactam
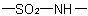 as a part of a ring are named as heterocycles or in accordance with
R-5.7.5.2 using "sultam" in place of "sultone". The locant for the point of attachment of the sulfonyl group is cited first and, where there is a choice, has preference over the imino group
for lower locant.
as a part of a ring are named as heterocycles or in accordance with
R-5.7.5.2 using "sultam" in place of "sultone". The locant for the point of attachment of the sulfonyl group is cited first and, where there is a choice, has preference over the imino group
for lower locant.
Examples to R-5.7.5.4


Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact webmaster@acdlabs.com