
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

641.2 - When another group is also present that has priority for citation as principal group, organic oxy acids of sulfur are named by adding to the name of the parent compound the appropriate prefix given in Table XII.Examples to Rule C-641.1
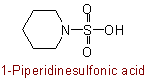
(cf. Rule C-661.1)
Exceptions:

(cf. Rule C-641.7)
641.3 - Organic acids derived from an oxy acid named according to Rule C-641.1 and in which one, two, or three atoms of oxygen of the acid group have been replaced by sulfur are named by placing "thio-", "dithio-", or "trithio-" immediately before the appropriate affix for the oxygen analogue given in Table XII.Examples to Rule C-641.2
Note: A compound R-SnH is named as a hydropolysulfide if the structure of the sulfur-containing group is unknown (see Rule C-513.1) and as a polysulfane derivative if the presence of an unbranched chain of sulfur atoms is to be stressed (see Rule C-515.2). In the latter case, the sulfane nomenclature may also be applied to compounds having structures such as R-Sn-OH.Examples to Rule C-641.3
641.4 - The two forms of a monothiosulfinic, monothiosulfonic, or dithiosulfonic acid in which hydrogen is attached to oxygen or sulfur may be differentiated when required by use of S-acid for the form -S(O)-SH and O-acid for the form -S(S)-OH.
641.5 - For the nomenclature of esters and salts of sulfur acids the ending "-ic acid" is changed to "-ate" (see Rule C-84.1), the resulting word being preceded by the name of the cation or the radical.Examples to Rule C-641.4
641.6 - When a group having priority for citation as principal group is also present, esters of sulfur acids are designated by composite prefixes formed from the following group names: RS-, alkoxy, etc.; RS-, alkylthio, etc.; -S(O)-, sulfinyl; -S(O)2-, sulfonyl; -S(S)-, thiosulfinyl; -S(S)(O)-, thiosulfonyl.Examples to Rule C-641.5
641.7 - Radicals derived from an organic oxy-sulfur acid by loss of a hydroxyl group are named by changing the syllables "-ic acid" to "-yl". (For the difference between names of the types benzenesulfonyl and phenylsulfonyl, etc., see Note to Rule C-631.1 andcompare Rules C-403.2, C-543.3, C-641.8, and C-641.9).Examples to Rule C-641.6
641.8 - (a) Amides derived by substituting NH2 for OH in an organic sulfur acid are named by changing the syllables "-ic acid" to "-amide", or as acyl derivatives (see Rule C-641.7) of the amine (compare Rule C-822; see also Note to Rule C-631.1).Examples to Rule C-641.7
Exceptions:
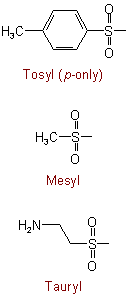
(from Taurine; see Rule C-641.1)
Exception:Examples to Rule C-641.8(a)
N-Phenyl-substituted amides may be named with "anilide" in place of N-phenylamide, locants for substituents in the aniline residue being primed, as in:
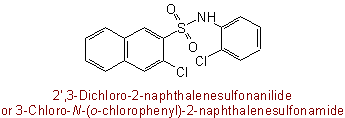
(b) When a group having priority over the amide group for citation as principal group is also present, the groups -SO2-NH2, -SO-NH2, and -S-NH2 are designated by the prefixes "sulfamoyl-", "sulfinamoyl-" and "sulfenamoyl-", respectively. Substituents on the nitrogen atom are cited as N-prefixes, and replacement of oxygen by sulfur is denoted by thio prefixes as described in Rule C-641.3 (see Note to Rule C-631.1).
(c) A radical formed from the amide of a sulfur acid by loss of one hydrogen atom from the NH2 group is designated by changing the ending "-amide" to "-amido-", the complex radical name being used as a prefix.Examples to Rule C-641.8(b)
641.9 - Other nitrogen derivatives of organic sulfur acids are named as described in Subsections C-8 and C-9 for similar derivatives of carbon acids, but with "-carbox-" replaced by "-sulfon-", "-sulfin-", or "-sulfen-" before a vowel, and "-carbo-" replaced by "-sulfono-", "-sulfino-", or "-sulfeno-" before a consonant, as appropriate.Examples to Rule C-641.8(c)
641.10 - As exceptions, because of precedent, hydroxy-derivatives of benzene and hydroxy or amino derivatives of naphthalene (but not similar derivatives of other cyclic compounds), when substituted also by -SO3H groups, may be named phenolsulfonic, naphtholsulfonic, and naphthylaminesulfonic acids, respectively; the hydroxyl or amino group(s) have priority for lowest available numbers. Derivatives of these acids may be named and numbered similarly. Nevertheless, the systematic names are preferred.Examples to Rule C-641.9
Note: Benzenesulfonamidine is not to be used.
See Recommendations'93 R-5.7.2, R-3.4Examples to Rule C-641.10


Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact webmaster@acdlabs.com