
ACD/Name (Chemist Version) offers a standardized set of features for quick and simple generation of IUPAC names, and structures from names. It is a streamlined version of our popular ACD/Name software.
View a full description and pricing on our web store.

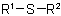 have the generic name "sulfides" Specifically they are named analogously to ethers, in
substitutive nomenclature with "alkylthio-" or "arylthio-", etc., in place of "alkyloxy-", etc., in radicofunctional nomenclature with "sulfide" in place of "ether" or "oxide", and
for assemblies of identical units with "thio-" in place of "oxy-".
have the generic name "sulfides" Specifically they are named analogously to ethers, in
substitutive nomenclature with "alkylthio-" or "arylthio-", etc., in place of "alkyloxy-", etc., in radicofunctional nomenclature with "sulfide" in place of "ether" or "oxide", and
for assemblies of identical units with "thio-" in place of "oxy-".
514.2 - When nomenclature according to Rule C-514.1 becomes complex, as, for example, when several sulfur atoms are present in an acyclic compound, replacement nomenclature can be used (see Subsection C-0.6).Examples to Rule C-514.1
514.3 - Cyclic sulfides are named by the following methods: (a) by prefixing "thia-" to the name of the parent carbocyclic compound; (b) by using an extension of the Hantzsch-Widman method (Rule B-1); or (c) by trivial or semitrivial names for cyclic sulfur compounds set out in Rule B-2.11.Example to Rule C-514.2
514.4 - A sulfur atom directly attached to two carbon atoms already forming part of a ring, or to two adjacent carbon atoms in a chain, may be indicated (a) by the prefix "epithio" or (b) by means of Rule C-514.3(a).Examples to Rule C-514.3
See Recommendations'93 R-5.5.1.2Examples to Rule C-514.4
Note, however


Published with permission of the IUPAC by Advanced Chemistry Development, Inc., www.acdlabs.com, +1(416)368-3435 tel, +1(416)368-5596 fax. For comments or suggestions please contact [email protected]