August 23, 2023
by Baljit Bains, Marketing Communications Specialist, ACD/Labs
Mass spectrometry (MS) is a powerful analytical tool that enables scientists like you and me to identify and quantify molecules with remarkable precision. In A Beginner’s Guide to Mass Spectrometry, you will have learned the basic principles of MS, the components of mass spectrometers, and how these components work, along with the steps involved in MS.
The steps involved in MS include ionization, ion separation by a mass analyzer, detection, deflection, and data processing. Understanding the different ionization methods in mass spectrometry is crucial, as the ionization process is the heart of MS.
What is Ionization in Mass Spectrometry?
Ionization is the process of converting neutral molecules into charged ions for analysis. The original sample can be solid, liquid, or gas. In cases where samples are solid or liquid, they are transformed into a gaseous state and then subjected to ionization through an ion source. This process involves the loss of an electron, leading to the formation of a positively charged cation.
The ionization chamber is maintained under vacuum conditions to prevent/minimize interaction with airborne molecules. A positively charged metal plate facilitates the movement of the samples to the next part of the apparatus.
Spectrometers work in either positive or negative ion mode, and it is crucial to select the most appropriate setting for accurate data analysis. The method of ionization plays a critical role in interpreting mass spectra.
Understanding Mass Spectrometry Ionization Methods
Ionization methods can be categorized into two groups: hard ionization and soft ionization.
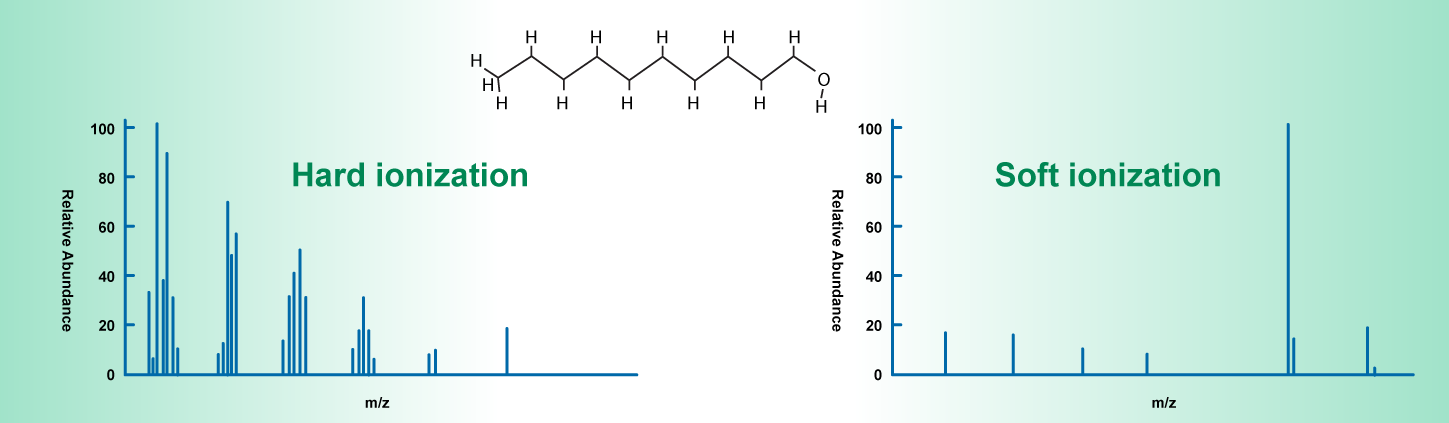
There are a variety of ionization techniques available, and the technique used depends on the experimental goals and sample characteristics. To learn more about how the ionization source impacts the interpretation of mass spectra, read Common Adduct and Fragment Ions in Mass Spectrometry.
Hard Ionization Techniques
Hard ionization techniques involve applying excessive energy to the sample during ionization, leading to sample fragmentation. During this process molecules lose an electron (become ionized) and become highly excited. As they relax, extensive fragmentation occurs resulting in numerous positive ions with various masses less than the mass of the molecular ion.
Electron Ionization (EI)
EI was one of the very first mass spectrometry techniques developed. It is a popular hard ionization method in which high-energy electrons produce ions after interacting with atoms or molecules in a solid or gas phase. Since it uses high energy to generate ions, it creates a lot of ion fragmentation which can be helpful for determining the structure of unknown compounds. When EI is combined with other separation techniques, it can be used to detect other thermally stable and volatile compounds in solid, liquid, and gas states. EI has high ionization efficiency and sensitivity, and it can provide a lot of structural information.
- Analysis: Useful for organic compounds with molecular weights below 600 Da
- Detection: EI can only detect positive ions
- Applications: EI has many different applications including, but not limited to, environmental analysis, archaeological analysis, forensic analysis, and pharmaceutical analysis
Inductively Coupled Plasma (ICP) ionization
ICP is a hard ionization technique used to measure elements at trace levels in biological fluids at atmospheric pressure. ICP ionizes the sample via an inductively coupled plasma (usually argon plasma) coupled to energy via an induction coil. The sample must be in either gas or vapor form, which then decomposes into its elements and these elements are transformed into ions. These ions can be detected and stored based on their mass.
- Analysis: Useful for detecting trace levels of metals and non-metals in liquid samples
- Detection: Metals and non-metals in liquid samples at low concentrations and different isotopes of the same element
- Applications: Trace element analysis in the clinical laboratory, as well as environmental, pharmaceutical, and geochemical analysis
Soft Ionization Techniques
Soft ionization techniques, apply less energy to ionize the sample, resulting in minimal fragmentation. Here, the spectra contain mainly the most abundant molecular ion peak.
Atmospheric Pressure Chemical Ionization (APCI)
APCI is a soft ionization technique that uses gas-phase ion-molecule reactions at atmospheric pressure to produce primary ions on a solvent spray. It is commonly coupled with high-performance liquid chromatography (HPLC). APCI produces a singly charged product and although this avoids signal overlap, it gives limited structural information due to the production of few fragment ions.
- Analysis: Molecular weight less than 1500 Da
- Detection: Polar and thermally stable non-polar compounds
- Applications: Analysis of drugs, pesticides, various organic compounds, and nonpolar lipids
Atmospheric Pressure Photo Ionization (APPI)
APPI is a soft ionization technique often coupled to liquid chromatography (LC). It uses photochemical action to ionize samples in the gas phase. The solvent and the sample from liquid chromatography form a gaseous analyte which is then ionized to interact with photons emitted by the light source at atmospheric pressure. The APPI light source can be an argon lamp or more frequently a xenon lamp. The ions are then introduced into the mass spectrometer for analysis. It can simultaneously ionize polar and non-polar small molecules, so more compounds can be analyzed in a single pass.
- Analysis: Samples in gas phase
- Detection: Weakly polar and non-polar compounds
- Applications: APPI is used for analyzing pesticides, steroids, and drug metabolites lacking polar functional groups. It is being extensively deployed in security applications for explosives detection.
Electrospray Ionization (ESI)
ESI is a soft ionization method using electrospray to apply a high voltage to a liquid to produce an aerosol, producing multiply charged ions. Using ESI, you can choose from positive ion mode or negative ion mode. In ESI, higher molecular weight molecules tend to carry multiple charges, the distribution of charge states accurately quantifies molecular weight, resulting in accurate molecular mass and structural information. The experimental parameters and choice of solvent used must be carefully selected.
The process of electrospray ionization consists of three stages:
- Droplet formation is where the sample solution is ejected from the capillary to form a charged droplet in the presence of a high-voltage electric field.
- Desolvation is when the droplets enter the spray chamber and are evaporated by the countercurrent of the heated dry gas, the diameter of the droplets gets smaller, and the surface charge density increases. When the repulsive forces between the charges are enough to counteract the surface tension of the droplet, the droplets will split into smaller charged droplets.
- Then there is gas phase ion formation. Here the size of the charged droplets decreases, and the ions turn into gas phase ions.
Coupling ESI with tandem mass spectrometry (ESI-MS/MS) helps to overcome the obstacle of obtaining very little structural information from the simple ESI mass spectrum. ESI can also be coupled with high-performance liquid chromatography (HPLC) for the analysis of both small and large molecules. ESI has improved sensitivity and accuracy over other MS techniques, allowing for a broadened application in the protein field.
- Analysis: Both small and large molecules of various polarities in more complex biological samples
- Detection: Analysis of large non-volatile molecules, inorganic substances, organometallic ion complexes, and biomacromolecules
- Applications: Analysis of peptides, proteins, and nucleotides.
Chemical Ionization (CI)
CI is a soft ionization technique that uses a reagent gas to ionize sample molecules through ion-molecule reactions in the gas phase. Samples to be analyzed must be in vapor form or vaporized before being introduced to the ion source. The first step is for the reagent gas to undergo electron ionization, generating a molecular ion. This molecular ion will then fragment as it reacts with other reagent gas molecules and ions, creating analyte ions. It is important to select the most appropriate reagent ion as it increases the selectivity and response time of CI.
Due to having little excess energy from the ions, there is little fragmentation that occurs, and resultantly, little structural information is obtained. Because there are few fragments formed, the products are usually ions of the analyte, which makes it possible to get the exact molecular weight of the analyte.
- Analysis: Both small and large molecules of various polarities in more complex biological samples
- Detection: Molecules that typically fragment a lot under EI conditions
- Applications: Identification, structure elucidation, and quantification of organic compounds, and some use in biochemical analysis
Matrix-Assisted Laser Desorption/Ionization (MALDI)
MALDI is a soft ionization technique that creates ions from large molecules with minimal fragmentation using a laser energy-absorbing matrix. The sample is prepared and ionized before analysis with the mass spectrometer.
The MALDI method consists of 3 steps:
- A large quantity of suitable matrix material is mixed in with the sample and applied to a metal plate. The matrix must coexist with the sample and ensure minimal interaction between the molecules of the sample.
Choosing the matrix is the most crucial step of the MALDI analysis. The ideal matrix should have strong electron absorption at the laser wavelength being used, lower vapor pressure, better stability in a vacuum, and be miscible with the solid analyte. Matrices are commonly solid and organic, but they can produce background peaks that can interfere with the characterization of the sample compounds. Compounds that have been shown to reduce background interference include inorganic materials, porous silicon, and surfactant-inhibiting substrates. - A pulsed laser (usually a nitrogen laser) exerts high-intensity energy on the sample/matrix material mix so that it can desorb from the metal plate with minimal fragmentation. The matrix will absorb the UV light and convert it into heat energy. A small part of the analyte/matrix will rapidly heat and vaporize. The matrix absorbs the UV light to protect the analyte from being damaged and transmits that energy to the sample to vaporize and ionize the sample.
- The analyte molecules either protonate or deprotonate, creating ions that can then be analyzed by the mass spectrometer. This transferring of protons is a gentler and less destructive technique.
It produces a single mass spectrum which is helpful for analyzing multi-component samples. It can be coupled with Fourier transform ion cyclotron resonance mass spectrometry and resolution is further improved (FTICR-MS).
Most commonly, MALDI is coupled with a Time-of-Flight mass spectrometer (TOF-MS) in which the ions become separated based on their individual masses and charges as they travel the length of the machine. Once they reach the end of the spectrometer they can be detected, and data collected.
- Analysis: Polar, non-volatile, and thermally unstable samples
- Detection: Analyze biomolecules (DNA, proteins, peptides, carbohydrates) and organic molecules as these tend to be more fragile and more likely to fragment when ionized by other ionization methods
- Applications: an analytical tool in pathology to identify and quantify proteins, peptides, drugs, and metabolites
In mass spectrometry, ionization plays a pivotal role in turning molecules into charged ions, setting the stage for their analysis and characterization. Understanding ionization methods enables selection of the most appropriate technique to accurately analyze your data.
To refresh your knowledge about the basic principles of MS, read our previous blog post: A Beginners Guide to Mass Spectrometry.
You can learn more about ACD/Labs mass spectrometry software tools here.


