August 10, 2023
by Sanji Bhal, Director, Marketing & Communications, ACD/Labs
Why pKa Values are Relevant to Scientists in Pharma/BioTech
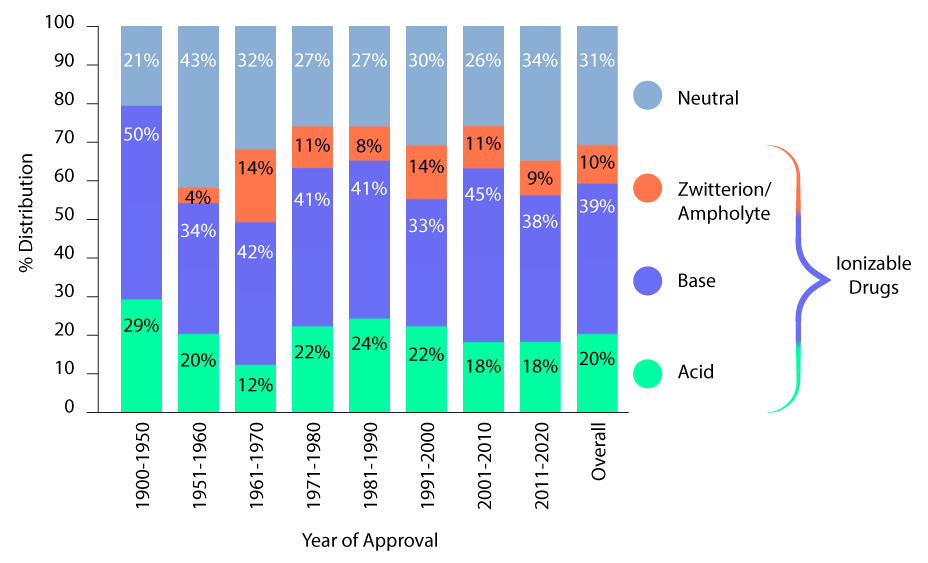
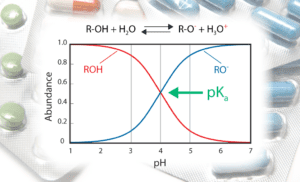 Many of the small molecules under investigation in pharmaceutical and biopharmaceutical labs are susceptible to ionization. While the composition of positively charged molecules (cations), negatively charged molecules (anions), and molecules with groups that are both positively and negatively charged (zwitterions) differs by therapeutic area and drug target class (the protein family being targeted); the vast majority are ionizable. A study by process development scientists at Amgen1 finds this to be true for 70% of the oral molecules approved by the FDA between 1900 and 2020. Furthermore, they report that this trend has not changed in the last 40–50 years.
Many of the small molecules under investigation in pharmaceutical and biopharmaceutical labs are susceptible to ionization. While the composition of positively charged molecules (cations), negatively charged molecules (anions), and molecules with groups that are both positively and negatively charged (zwitterions) differs by therapeutic area and drug target class (the protein family being targeted); the vast majority are ionizable. A study by process development scientists at Amgen1 finds this to be true for 70% of the oral molecules approved by the FDA between 1900 and 2020. Furthermore, they report that this trend has not changed in the last 40–50 years.
Ion Class Distribution of FDA-Approved Oral Molecules Over 120 Years
Ionization Considerations in Drug Discovery and Development
An understanding of ionization is critical. From screening assays in early discovery to identifying a suitable lead series, to the final formulation of drug product to ensure delivery to patients in a stable form, ionization influences important decisions.
What pKa Reveals in Screening & Lead Optimization
In vitro biological assays are used to screen molecular entities for activity against a chosen target. These assays require strict pH and osmolarity control to ensure that their constituents function as they would in vivo. Predominantly solution-based, assays are buffered to achieve a functional pH.
By predicting pKa for each test material, ionizable functional group(s) are revealed and scientists can review the overall charge state (apparent pKa) and microspecies present in the assay to focus on ‘lead-like’ or ‘drug-like’ compounds. This data early in the process can significantly impact decisions to shorten lead to candidate timelines and produce novel analogs that address ADMET (absorption, distribution, metabolism, excretion, toxicity) deficiencies:
- When ionization is seen to influence/modulate the bioactivity of an entity or entities, it helps focus lead optimization efforts on that lead series/molecular entities
- If charge state differences do not influence bioassay results within a lead series, medicinal chemists may be able to modify the structure to enhance off-target properties such as ADME/Tox without impacting potency (inhibition, agonism, degradation, etc.)
- If ionizable groups are not seen to contribute to potency, it gives project teams the freedom to make changes to that part of the molecule to make the molecule ‘more druggable’ (e.g., improve solubility, modulate dissolution rates, etc.)
- When localized charge is a potency-meditating factor, medicinal chemists know to protect that part of the molecule from modification which can reduce design-make-test-analyze (DMTA) cycles
pKa in Drug Formulation
Ionization and related molecular characteristics also impact formulation. Since pH adjustment is a powerful way to enhance solubility and dissolution, salt formation is an effective strategy for clinical development. Knowledge of pKa helps scientists identify suitable formulations for delivery and select stable physical forms (salt formation through pH modification).
Other Applications of Ionization Data in Pharmaceutical R&D
pKa determines ionization behavior in many environments, not only in vivo but also in daily work carried out in R&D laboratories.
Ionization Descriptors and Structure Activity Relationships (SAR)
Ionization has a direct impact on SAR. It relates to aqueous solubility and the distribution coefficient, logD (a measurement of the lipophilicity of ionizable compounds) and ADME/Tox properties—absorption, bioavailability, volume of distribution, permeability, hERG toxicity, and drug clearance. Computational chemists and modelers rely on accurate ionization calculators to model these complex in vivo behaviors. Learn more about how ionization influences ADME/Tox parameters.
Ionization in Chromatography
Chromatographers and separation scientists use pKa and related logD values to optimize HPLC and UHPLC separations. An understanding of the ionic form(s) of the analyte(s), and the pH of the mobile phase helps them choose the most appropriate pH (buffer solution) and stationary phase (column) for separations. Using physicochemical properties to design robust chromatographic methods is part of a quality by design (QbD) approach to method development and optimization. Learn more about mobile phase pH & analyte pKa to improve chromatographic separations.
Ionization in Synthetic Chemistry
Acid/base strength or the order of acidity/basicity is used by synthetic chemists (medicinal chemists and process chemists) to predict/understand how reactions occur, and to select appropriate reagents for syntheses. Knowing the pKa of a carboxylic acid, for example, allows them to select the correct base for that synthetic step. When there is more than one labile (acidic) proton, an understanding of pKa values allows the scientist to select reactivity around a molecule.
Find tables of pKa values in various media here.
Software for Ionization Prediction
In silico calculation of the acid dissociation constant (pKa) has long been an alternative to physical experiments. A reliable pKa calculator can negate the need for hundreds or thousands of measurements, saving time, materials, and human effort. This not only helps make projects more efficient and productive, it also supports green chemistry initiatives and sustainable practices. Scientists can use predicted pKa values to make data-driven decisions throughout pharmaceutical discovery and development. Calculated ionization data can be provided to researchers ‘on-demand’ or integrated into other applications. Learn about ACD/Labs’ acid dissociation calculator.
- P. Agarwal, J. Huckle, J. Newman, D.L. Reid. (2022). Trends in small molecule drug properties: A developability molecules assessment perspective. Drug Discovery Today, 27(12), 103366. https://www.sciencedirect.com/science/article/abs/pii/S1359644622003592


