September 19, 2023
by Baljit Bains, Marketing Communications Specialist, ACD/Labs
Selection of Mobile Phase pH for the Best Separations
Chromatography aims to separate an analyte mixture into sharp, symmetrical, and well-resolved peaks for quantitative and qualitative analysis. This requires satisfactory separation of components, with suitable resolution within a reasonable time frame. To determine the best starting conditions, it is crucial to understand ionization and the ionic form(s) the analyte is at a given pH. The use of predictive software tools allows selection of optimal conditions and helps build robustness into methods from the start.
The Importance of Ionization in Chromatography
To determine the best separation conditions, particularly mobile phase pH, it is essential to understand the behavior of ionic form(s) of analytes and account for physicochemical properties (like pKa and logD). Chromatographic resolution between two or more peaks depends on the separation efficiency (N), analyte retention (k), and separation selectivity (α). For ionizable analytes, pH drastically affects each of these factors.
Effects of Mobile Phase pH on Acids & Bases
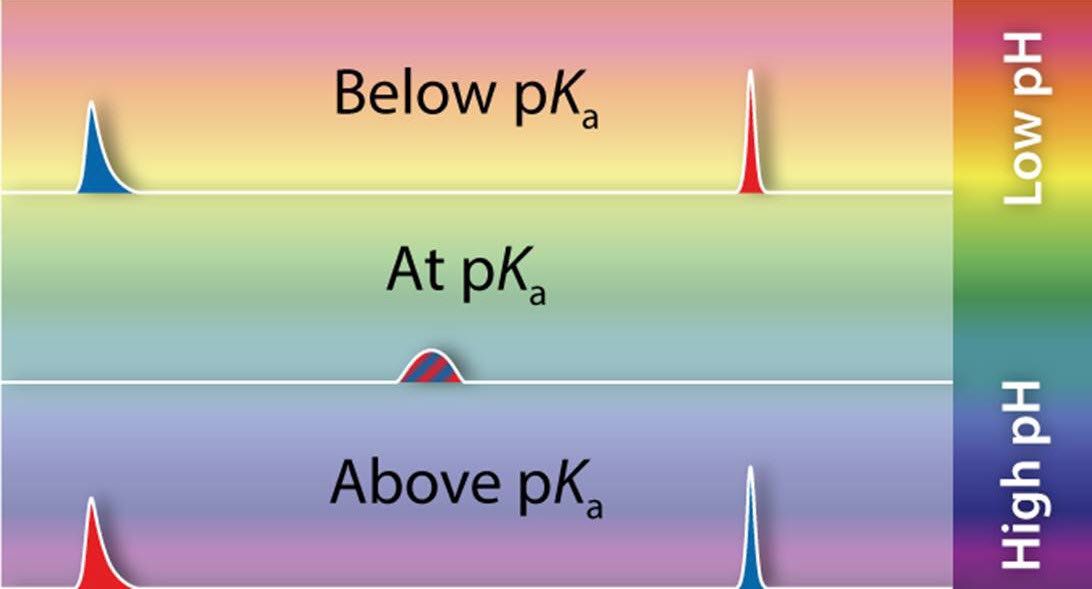
What is the Degree of Ionization?
The degree of ionization (pKa) refers to the strength of the acid/base and controls the ionization state of an analyte when in solution. It predicts how easily a molecule will donate or accept a proton at a specific pH. Changing the solution’s pH can impact the state of ionization and the distribution of ionized forms. The ionization state of the analyte directly affects the degree of interaction between the analyte and the stationary phase. Knowing the pKa of analytes will help select an appropriate and robust mobile phase pH and offer flexibility in method optimization.
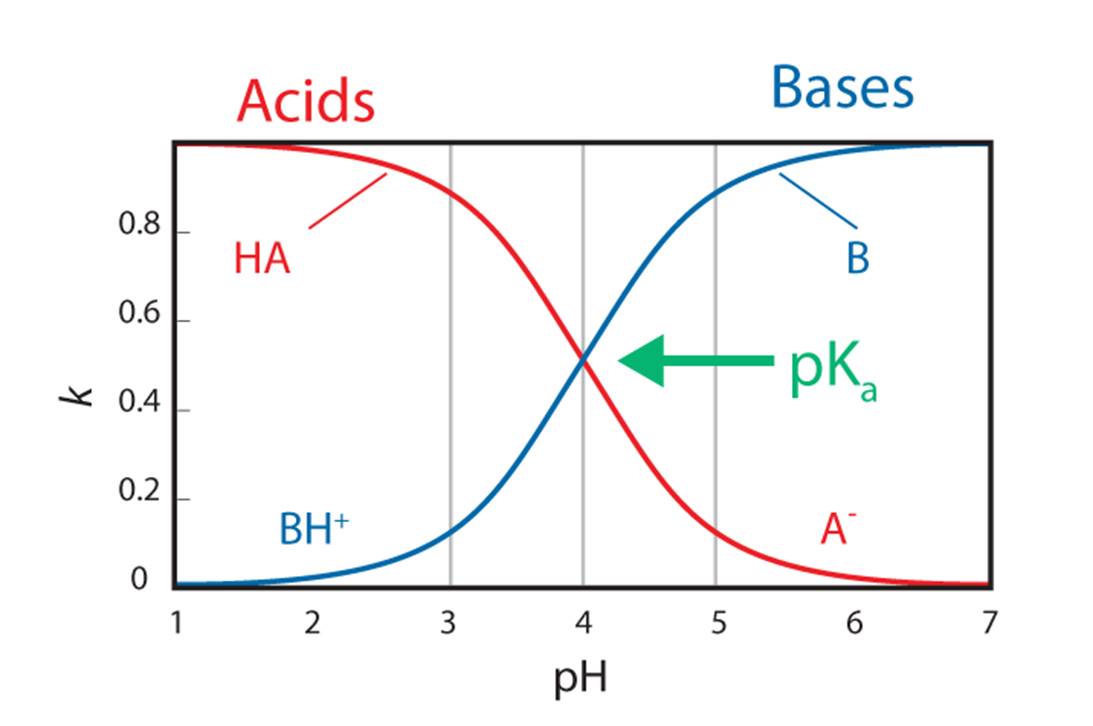
The Power of Mobile Phase pH
Mobile phase pH is a powerful tool that can be used to control analyte retention, selectivity, and peak shape. Predictive tools can help determine the optimum mobile phase pH, ensuring method robustness and reproducibility.
To determine the most appropriate mobile phase pH, you must consider the following factors:
- Dominant Ionic Form—For a mixture of several ionizable analytes of interest, having all the analytes in the same form (either ionized or unionized) is preferred and should be considered when selecting the desired pH
- Hydrophobicity (non-polarity)—In reversed-phase liquid chromatography (RPLC), analytes that are more polar tend to have shorter retention times and elute before those that are non-polar
For good chromatographic separations, it is essential to minimize peak abnormalities where possible. Mobile phase modifiers like buffers and organic modifiers can be used to maintain the desired optimum pH and fine-tune peak shape. Tight control of the pH helps to separate closely eluting or overlapping peaks.
Putting Theory into Practice—Mobile Phase pH Selection in the Separation of Sulmetrim
To achieve HPLC/UPLC separations, it is necessary to understand the ionic form(s) of the analyte at a given mobile phase pH. The effects of pH variation on analyte retention can be seen in Sulmetrim (trimethoprim-sulfamethoxazole)—a compound used to treat bacterial infections of the respiratory tract, urogenital tract, and alimentary tract.
Here, the distribution of ionic forms depending on the pH is calculated using pKa and logD.
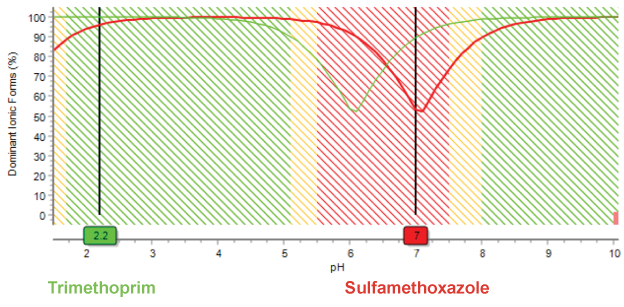
At acidic pH (2.2), both trimethoprim and sulfamethoxazole are present in a dominant form. At neutral pH (7.0), comparable portions of sulfamethoxazole ionic forms are in equilibrium. As ionic forms change, there can be a negative impact on the symmetry and width of chromatographic peaks. The pH where there is a better chance of peak separation and less likely to be peak shape issues in indicated by green. The red color indicates pH to be avoided.
How Variations in Mobile Phase pH Impact Peak Shape and Analyte Selectivity
Variations in mobile phase pH can significantly affect peak symmetry and width, and peak spacing (selectivity). When the mobile phase pH is too close to the analyte’s pKa, both species will be present in the sample, causing the peaks to be split or have shoulders. Small changes in pH can have a huge effect on separation selectivity, especially in the presence of similar structures.
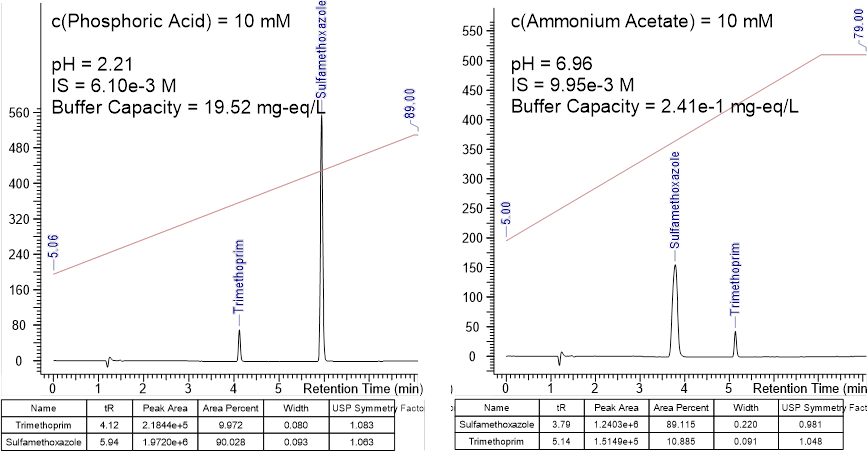
Here we see the chromatograms of trimethoprim and sulfamethoxazole in acidic and neutral conditions. Under acidic conditions, the peaks of both components on the chromatogram have suitable symmetry and width. However, as the pH moves towards neutral (pH 7), while trimethoprim retains its shape, the sulfamethoxazole peak has widened and moved to the left of trimethoprim.
How Variations in Mobile Phase pH Impact Analyte Retention
Retention behavior also changes with ionization. Slight changes in pH, especially when close to the analyte’s pKa can lead to drastic changes in retention time. Ion suppression (lowering pH for acidic analytes or raising pH for basic analytes) will notably lengthen retention.
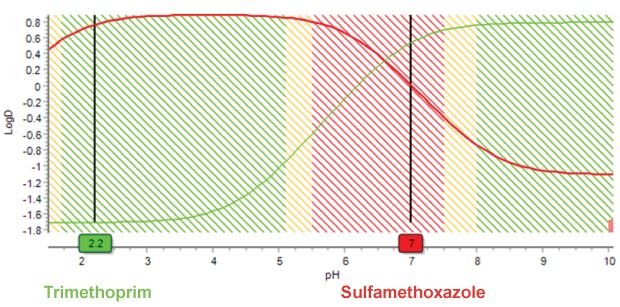
This plot of pH vs. logD illustrates changing lipophilicity and hydrophobicity with pH due to ionization. With increasing pH, the hydrophobicity of trimethoprim increases, and the hydrophobicity of sulfamethoxazole decreases. Since retention is dependent on hydrophobicity, this explains the change in order of peak elution.
Using Prediction Tools to Ensure Separation Robustness
While it is ideal to use known analyte structures, this is not always possible. When dealing with unknown structures, a good starting point can be to screen the sample(s) on a generic gradient with low and high-pH mobile phases. Problems in retention behavior, selectivity, and peak shape can be due to poor selection of chromatographic parameters (like pH and pKa). This can be prevented by taking a systematic, rational approach to method development. This involves recognizing the extent to which the pH or pKa can be varied while still maintaining satisfactory analytical results.
Predictive tools in software like AutoChrom® and Method Selection Suite can help establish how much variation of mobile phase pH is possible, while still ensuring sufficient separation and resolution. These tools include relevant physicochemical property prediction (e.g., pKa, logD) and use this information in simulating methods to determine optimum conditions. Using predictive tools not only helps with effectively starting a method development project but also in method refinement—to resolve peak splitting issues and identify optimum conditions more quickly while building robustness into methods from the start.
You can find out more about ACD/Labs’ method development tools here.


