Improved IUPAC Naming for Cyclic Peptides and Acyclic Heterones
- You can now generate IUPAC names for cyclic peptides with internal amido groups and disulfide bridges
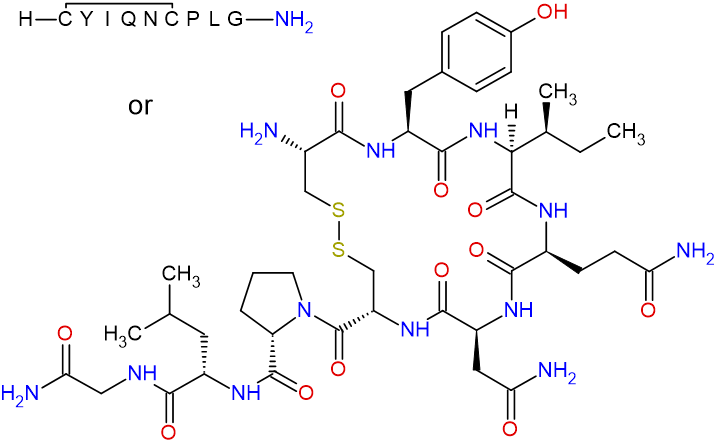
Oxytocin
Previous: 1-{(4R,7S,10S,13S,16S,19R)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-[(2S)-butan-2-yl]-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carbonyl}-L-prolyl-L-leucylglycinamide
Current: S1,S6-cyclo(L-cysteinyl-L-tyrosyl-L-isoleucyl-L-glutaminyl-L-asparaginyl-L-cysteinyl-L-prolyl-L-leucylglycinamide)
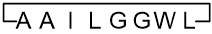
Previous: cyclo(L-alanyl-L-isoleucyl-L-leucylglycylglycyl-L-tryptophyl-L-leucyl-L-alanyl)
Current: 1,8-anhydro(L-alanyl-L-alanyl-L-isoleucyl-L-leucylglycylglycyl-L-tryptophyl-L-leucine)
- You can now generate names for acyclic heterones
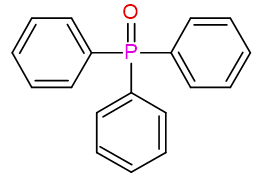
Previous: oxotri(phenyl)-λ5-phosphane
Current: triphenyl-λ5-phosphanone
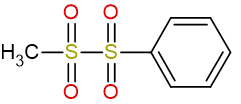
Previous: 1-methyl-1,1,2,2-tetraoxo-2-phenyl-1λ6,2λ6-disulfane
Current: 1-methyl-2-phenyl-1λ6,2λ6-disulfane-1,1,2,2-tetrone