December 21, 2023
by Baljit Bains, Marketing Communications Specialist, ACD/Labs
Compliance with ICH Q14 Guidelines Simplified with Software
The new ICH Q14 guidelines are a scientific and risk-based approach to analytical procedure development. They outline a methodical approach to developing analytical procedures to ensure procedures are robust and appropriate for the intended use.
With so much variation in the approach to analytical method development, the International Council for Harmonization introduced these guidelines for consistency and unity. The aims of the guideline include improving communication with regulators, expediting approvals, and better management of post-approval changes of analytical procedures.
Implementation of the guidelines minimizes the amount of effort across the analytical procedure life cycle while increasing the understanding of the procedure and enhancing confidence in the procedure’s performance.
Which ICH Q14 Approach to Take? Minimal vs. Enhanced Approach
The ICH Q14 guidelines introduce and distinguish between minimal (or traditional) and enhanced approaches. Depending on the requirements and context of the analytical procedure, either the minimal approach or some or all elements of the enhanced approach can be applied. It is important to note that following the enhanced approach is not a regulatory requirement.
The elements for each approach described in the ICH Q14 guidelines are listed below.
Minimal Approach
For a minimal approach to analytical procedure development, the following elements should be identified and included:
- Attributes of the drug substance/drug product that needs to be tested
- Appropriate technology and related instruments
- Evaluation of performance characteristics (i.e., specificity, accuracy, robustness, etc.) by conducting appropriate development studies
- Define the analytical procedure description including the analytical procedure control strategy (i.e., parameter settings and system suitability)
Enhanced Approach
An enhanced approach should include the elements for a minimal approach PLUS one or more of the following elements:
- Evaluating sample properties
- Defining the Analytical Target Profile (ATP)
- Identification of analytical procedure parameters that can impact the procedure’s performance by conducting risk assessment and evaluating prior knowledge
- Investigating ranges and interactions between analytical procedure parameters by conducting uni- or multi-variate experiments
- Ensuring adherence to performance criteria by defining an analytical procedure control strategy
- Defining a lifecycle change management plan by defining and reporting categories of established conditions (ECs), proven acceptable ranges (PARs), or method operational design regions (MODRs)
In line with a QbD approach, the enhanced approach is a systematic approach that starts with predefined objectives and emphasizes process understanding—ensuring the analytical procedure is well understood and demonstrated to be fit for the intended purpose.
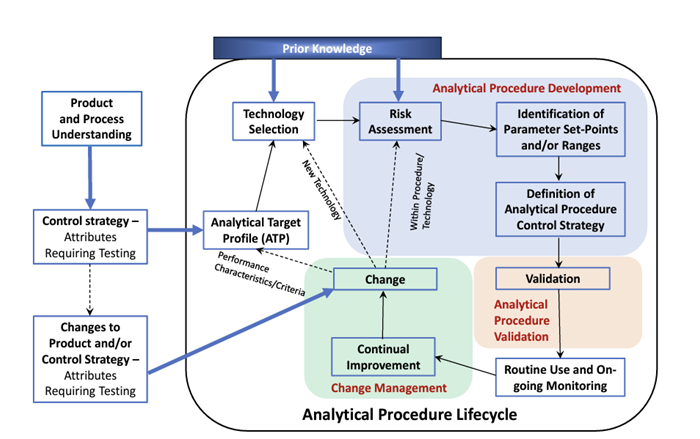
The Analytical Procedure Lifecycle
The analytical procedure lifecycle (shown in the figure below) highlights the different elements and how they are linked.
Elements of the Analytical Procedure Lifecycle
How AutoChrom Supports Implementation of the ICH Q14 Guidelines
Method development software, AutoChrom®, can help implement elements of the ICH Q14 guidelines. AutoChrom streamlines method development and helps you create robust methods faster. Tools and features within the software align with specific elements of the analytical procedure lifecycle.
Design and Develop Your Analytical Procedure
The Analytical Target Profile (ATP) forms the foundation for the analytical procedure. It consists of a description of the intended purpose of the analytical procedure, details to be measured, and relevant performance criteria. The ATP also assesses which technologies align with its criteria—helping to select the most appropriate technology. The ATP is maintained over the lifecycle—facilitating the procedure design and development, the subsequent performance monitoring, and continual improvement of the analytical procedure.
The strategy design feature in AutoChrom helps with procedure design and development. Using the software, you can screen databases of reference materials to define the starting point and build a strategy using the software wizard. The software allows you to follow the steps logically and rationally following QbD principles.
Manage and Apply Your Prior Knowledge
The development and subsequent lifecycle of the analytical procedure are dependent on knowledge management. Knowledge can be obtained from various sources including scientific or technical publications, established scientific principles, and/or proprietary experience.
AutoChrom can be used to:
- Document your decisions for data integrity
- Store the workflow alongside experimental data
- Upload data and decisions to a searchable and shareable database for future learning
- Create reports that document your method development project (including development strategy diagram, screening results, optimization results, method parameters, and more)
- Search prior knowledge to accelerate subsequent related projects
Minimize Risk
Risk management helps develop robust analytical procedures that mitigate the possibility of poor performance and minimize the likelihood of inaccurate results. Risk assessments can help to establish an analytical procedure control strategy.
AutoChrom helps to mitigate risk by:
- Automated screening and optimization trials—lowering your risk of transcription errors
- Modeling to visualize whether the method is within the desired performance region, which also ensures robustness
- Leveraging prior knowledge in your databases to make informed decisions about risk assessment
Hear how GSK is using in silico modeling for QbD method development and optimization in this webinar.
Improve Robustness with Built-in Workflows
Robustness is the ability of the analytical procedure’s capacity to meet the expected performance requirements when there are small changes. Prior knowledge and risk assessment are used to identify the parameters and appropriate associated ranges that must be experimentally investigated. Robustness studies test the variations of these parameters.
AutoChrom has built-in robustness studies that can be added to your strategy. The software allows you to vary method parameters and determine which are the best. Here is an example of a strategy that includes a robustness study.
- Set parameters (solvent ratio, column, pH, gradient, temperature, amount of buffer, and flow rate) and levels to be screened and optimized
- The software automatically generates a corresponding list of experiments
- Confirmation of robustness through simulation
Parameter Ranges of Analytical Procedures
Investigation of parameter ranges can provide additional knowledge about the analytical procedure’s performance. Using an enhanced approach, multi-variate experiments can be conducted to investigate the ranges for relevant parameters and their interactions. A method operational design region (MODR) consists of a combined range for two or more variables within which the analytical procedure demonstrates the fitness of the analytical procedure for its intended use.
Using AutoChrom, temperature/gradient resolution maps or suitability limit graphs can be created from optimization studies. The MODR can be displayed on these (shown in the figure below).
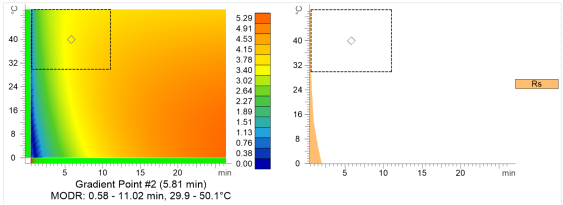
Resolution map and suitability limit graph created in AutoChrom, showing the gradient point, resolution (Rs), and MODR.
The MODR is the largest rectangle that can be created from the center point that meets suitability criteria. Using this as a guide and working within this region, a method should perform accurately.
Ensure the Analytical Procedure Performs Successfully with Suitability Criteria
The analytical procedure control strategy ensures that the analytical procedure performs as expected during routine use throughout its lifecycle. It consists of a set of controls, for example, analytical procedure parameters that need controlling or system suitability tests (SST).
Many factors can be adjusted in the software to optimize and create a robust method, including resolution, retention factor, gradient, and run time (shown in the figure below). There are also various visual cues (like color-coded resolution maps and dashed areas indicating regions that are not suitable) to inform you whether the suitability criteria have been met—helping you to generate and execute an accurate method.
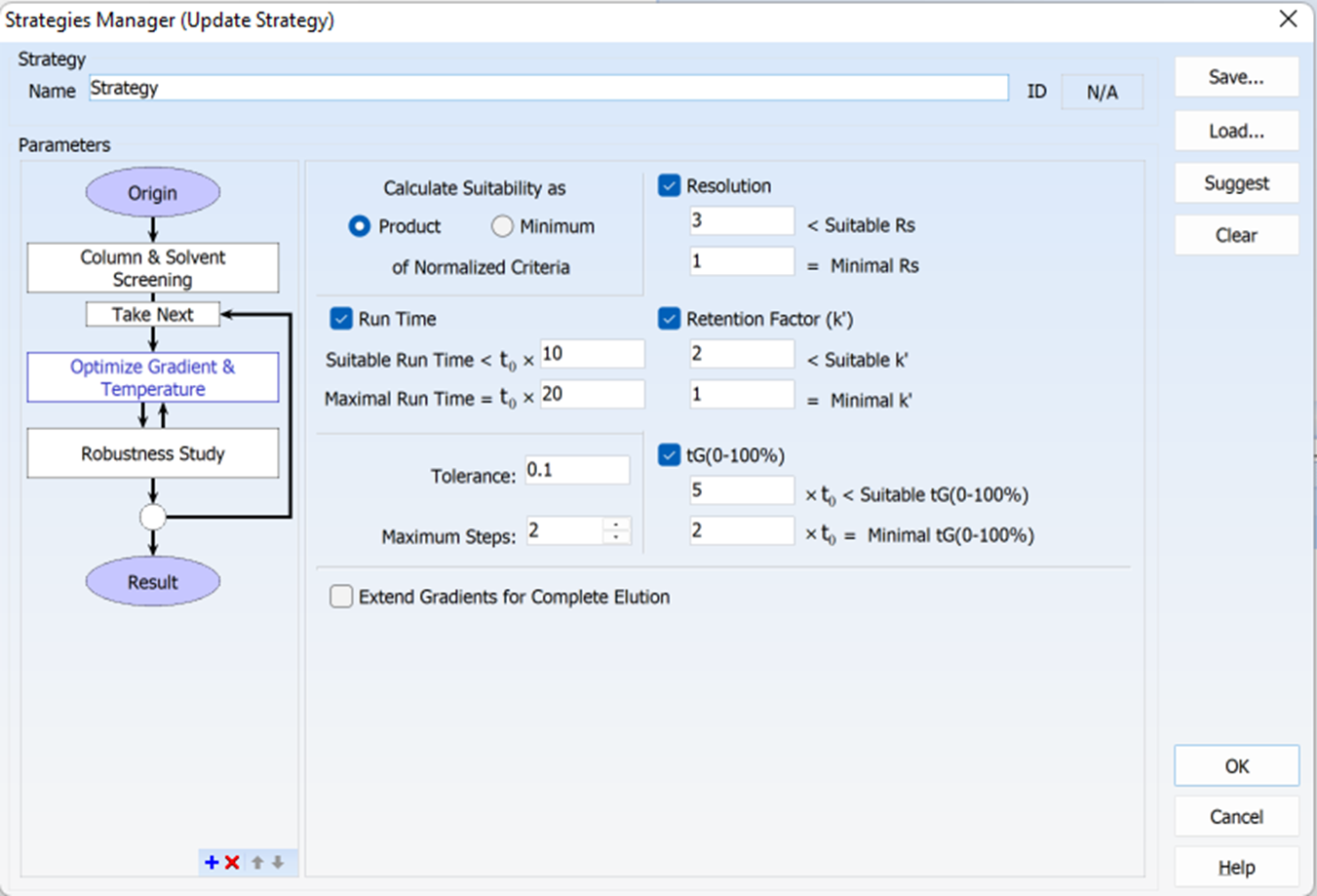
An example of the Strategies Manager window, showing the strategy parameters as well as the suitability criteria (run time, resolution, retention factor, gradient).
AutoChrom takes a scientific and risk-based approach to analytical method development, ensuring methods are robust and appropriate for the intended use. With an assortment of features and tools that follow QbD principles, it assists with procedure design, knowledge management, risk mitigation, and robustness. AutoChrom ensures you are well-equipped to design, understand, and control your analytical procedures. In line with ICH Q14 guidelines, you can continually monitor and update your processes for consistency and stability.
Learn more about AutoChrom here and get tips on implementing method development software in this post in which DOW scientists discuss their experience.
Reference: ICH Q14 Guidelines https://database.ich.org/sites/default/files/ICH_Q14_Document_Step2_Guideline_2022_0324.pdf