August 21, 2025
by Sarah Srokosz, Marketing Communications Specialist, ACD/Labs
What is coupling in 1H NMR?
In 1D 1H NMR, coupling (also known as J-coupling or spin-spin coupling) refers to the through-bond interaction between the magnetic moments of neighboring non-equivalent protons.
The most common couplings that are observed are those between vicinal protons (i.e., those between H atoms on neighboring C atoms).

Why does 1H–1H coupling matter?
In organic small molecules, these vicinal non-equivalent protons are common and result in predictable effects on the appearance of the relevant signals in a 1H NMR spectrum. Being able to recognize and decode this coupling information in a spectrum helps elucidate structures easier and faster.
How can you use proton–proton couplings to infer structural information?
Proton–proton coupling in a 1H NMR spectrum provides two important pieces of information in the resulting NMR signal—the multiplicity and the coupling constant.
What is multiplicity in 1H NMR?
The coupling interaction results in the splitting of an NMR signal into groups of symmetric (sub-)peaks with the intensities obeying Pascal’s triangle. The appearance of these signals is known as the splitting/coupling pattern or multiplicity. You may also see signals with any degree of coupling generically referred to as multiplets.
What does multiplicity tell us?
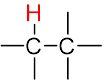
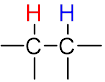
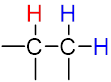
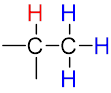
The multiplicity of a signal gives us information about the number of non-equivalent protons on nearby atoms that are involved in the coupling interaction according to the n + 1 rule.
| The n + 1 rule: The proximity of “n” equivalent H on neighboring carbon atoms causes the signals to be split into “n+1” lines. | ||||
 |
 |
 |
 |
|
| Multiplicity | 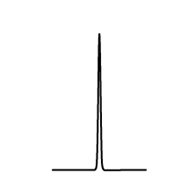 Singlet (s) Singlet (s) |
 Doublet (d) Doublet (d) |
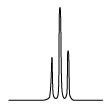 Triplet (t) Triplet (t) |
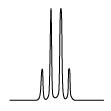 Quartet (q) Quartet (q) |
| Sub-peak ratio | – | 1:1 | 1:2:1 | 1:3:3:1 |
| n + 1 | – | 1 + 1 = 2 | 2 + 1 = 3 | 3 + 1 = 4 |
What are coupling constants?
The coupling constant (J) is the difference, typically expressed in Hz, between adjacent sub-peaks in a split signal, quantifying the strength of the coupling interaction. The coupling constant is independent of the field strength of the magnet used to acquire the spectrum.
What do coupling constants in 1H NMR tell us?
For a single coupling interaction, the coupling constant will be identical for both spins involved. This means that when one proton influences its neighbor through coupling, that neighbor exerts the same influence back with equal strength.
Therefore, by analyzing the observed coupling constants in a proton NMR spectrum, coupling partners can oftentimes easily be paired, which gives us information about the proton connectivity in the sample molecule.
By comparing the J-coupling for each splitting pattern in the spectrum below, roughly +/- 0.2 Hz, you can determine which multiplets pair up. Based on the pairing information, we can deduce that the proton is within proximity of its pair.
Coupled protons are:
Multiplets A and C = green line
Multiplets B and C = orange line
Multiplets D and C = pink line
Multiplets E and C = red line
Multiplets D and E = black line

Strong or Second-Order Coupling Can Also Help Match Coupled Signals
When the chemical shift of a pair of coupled protons is similar, second-order coupling may be observed. These second order effects (also known as tilting or roofing) produce distorted peaks in which the parts of the peak closest to each other become larger, while the outer parts become smaller. The signals appear to lean towards each other, which can also help identify neighboring protons in a spectrum.
The 1H NMR spectrum below illustrates an example of strong coupling among three multiplets. The peak intensities across Multiplets A and B are different, that is, the peak on the right side of the multiplet is higher in intensity than the peak on the left side. The purple arrow illustrates a tilt towards the right side for both multiplets. Multiplet C shows an opposite tilt, i.e., to the left side. Multiplets that tilt to form a roof are most likely related protons and thus are in proximity of each other. Therefore, one can say that Multiplets A and B are coupled to Multiplet C.

Although the field strength and coupling constants impact the amount of tilting, generally the closer the multiplets are, the more pronounced the tilting.
Complex Coupling in Proton NMR
When a proton has more than one neighboring non-equivalent proton, its signal is effectively split by each with their respective coupling constants. This results in complex splitting patterns being observed in the spectrum.
However, even in these complex cases, all the rules remain the same. We just have to account for the fact that the signal is split more than once. Consider the example below looking at two somewhat similar complex coupling patterns.
A Triplet of Doublets (td) vs. a Doublet of Triplets (dt)
The diagram below illustrates two coupling patterns: a triplet of doublets (td) and a doublet of triplets (dt). The J coupling constants for both multiplets are measured at 3.2 and 8.3 Hz.
Both have 1 coupling from 1 non-equivalent proton and 1 coupling from 2 non-equivalent protons (6 sub-peaks expected observed in both cases).
The difference is the left multiplet (td) is the result of a situation where the coupling constant from the 2-proton group is larger. In this case, it exhibits two couplings of 8.3 Hz and one of 3.2 Hz and the right multiplet (dt) is observed where the coupling constant from single proton is larger and so it exhibits one coupling of 8.3 Hz and two of 3.2 Hz.

Beyond Three Bonds: Identifying Meta Coupling in a 1H NMR Spectrum
While we have so far only considered 3-bond 1H–1H couplings, longer range interactions are possible, especially in aromatic systems. A relatively common example of this occurs in a substituted benzene ring, where aromatic protons that are in the meta position can exhibit coupling to each other. This is referred to as meta or 4J coupling. The coupling pattern is typically a doublet with a coupling constant of ~2 Hz.
Although a 2D COSY experiment can also provide information about such protons, one can save time by looking for this information in a 1H NMR spectrum first.
Illustrated below is a portion of a 1H NMR spectrum for a substituted benzene ring. Proton A is a doublet with a 2.3 Hz coupling and proton B is also a doublet with 2.2 Hz coupling. Proton A and B are coupled to each other due to the similar coupling constant (+/- 0.2 Hz). Another indication of coupling is the slight roof/tilt of the multiplets toward each other.

TIP: Be careful on the extent of line broadening applied to the FID, too much and the meta coupling information can be lost.
Why is there unexpected coupling in my spectrum?
There are cases where the perceived coupling constants can be misleading and thus wrong.
The 1H NMR spectrum below shows several multiplets for a major component, tryptophan, and a minor component.
For tryptophan, the aromatic multiplets (12 & 13) exhibit a dd pattern with a small coupling of ~2 Hz. The CH2 (6) exhibits a ddd pattern also with a small coupling of ~2 Hz.

If we look at the multiplets for the minor component, the signals exhibit the same splitting pattern with the same coupling constant of ~2 Hz.

One potential reason for what appears to be extra coupling constants is poor shimming. Shimming can affect line shape and peak resolution.
A special thanks goes to the Ontario NMR group hosted at UTSC.
1H NMR Coupling Exercise
When presented with a well-resolved NMR dataset, a highly experienced elucidator may be able to interpret the dataset through visual analysis—i.e., without any peak picking, integration, and multiplet analysis through software. In essence, this NMR interpretation skill equates to speed reading and can be learned with some practice.
The 1H NMR spectrum below displays 5 multiplets with the chemical shift and integration information removed. In this exercise, can you recognize which multiplets are coupled to each other?

The first step is to roughly estimate the integral values for each multiplet and thus eliminate any notion of overlapping peaks. From the 1H NMR spectrum, there are 5 multiplets roughly integrating to an equal area, i.e., the integral ratio is approximately 1:1:1:1:1.
The next step is to judge the coupling constants for each multiplet, i.e., the spacing between the peaks. Without getting too technical here, a good approach is to eyeball the coupling constants as small, medium, and/or large. From this point, it becomes a simple task of matching up the same couplings: A to B (small coupling), A to E (large coupling), and C to D (medium coupling).
Conclusion
When it comes to proton NMR, a large part of the information that can be deduced comes from the interpretation of the couplings. Being able to extract this information is a valuable skill for all chemists.
However, when the molecules in question become large and complex (such as natural products) or the NMR data is ambiguous, even the most skilled elucidators with all the NMR data they could gather (such as that of other nuclei and/or 2D spectra) can get stuck or end up at an erroneous structure.
In cases like these, automated structure verification (ASV) tools such as Spectrus Processor’s Single Structure Verification (SSV) can provide a helpful check of the proposed structure free of human bias. SSV compares the experimental spectrum to the predicted spectrum of the proposed structure, and evaluates the match using all available information, including couplings.
