October 1, 2018
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
5α-Cyprinol
Bile salts exist in the organisms of all certebrates. Their main function is to aid in the digestion of lipophilic compounds. 5α-Cyprinol sulfate (1) is one such bile salt which has earlier been found in fish species.
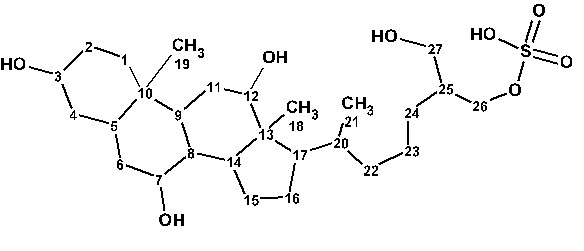
1
These type of samples are usually isolated in small quantities making detailed NMR characterization very challenging. They are usually characterized by various other spectrometric techniques while NMR data is rather scarce. Unsurprisingly there is very little NMR data in the literature for this particular compound, all of which have either incomplete assignments and/or low resolution 2D spectra. A consequence of this is that the existing complete 13C assignment is questionable.
Taking this into account, Hahn and coworkers [1] decided to confirm or amend the assignment of 1H and 13C resonances presented in literature. The substance was isolated again and carefully investigated. As a result the authors [1] revised the formerly published assignments. Moreover Hahn et al supplied in the article all NMR spectroscopy data in the fully machine readable, recently published NMReDATA format [2] for small molecule assignments.
5α-cyprinol sulfate was isolated from a methanolic extract of fish bile (Cyprinus carpio) after separation by preparative LC. The molecular mass and the presence of a sulfate group were revealed by ESI–MS/MS in the negative mode, and the molecular formula of the compound was determined as C27H48O8S. For the structure elucidation, authors acquired 1H, 1H –1H COSY, HSQC, HMBC, H2BC, 1D NOE and 1D TOCSY NMR data. Due to the low sample concentration the 13C chemical shifts were determined from 2D NMR experiments.
To challenge ACD/Structure Elucidator, we utilized the 1H, 13C, HMBC and COSY data which were measured by authors [1] and presented by them in the Supporting Information (see Table 1).
Table 1. 1D and 2D NMR spectroscopic data.
| Label | δC | δCclc* | CHn | δH | M | COSY | H to C HMBC |
| C 1 | 31.71 | 30.98 | CH2 | 1.42 | u | ||
| C 2 | 28.09 | 30.6 | CH2 | 1.64 | u | 1.42, 3.99 | |
| C 3 | 65.73 | 69.88 | CH | 3.99 | u | 1.51, 1.64 | C 5, C 1 |
| C 4 | 35.13 | 39.3 | CH2 | 1.32 | u | ||
| C 4 | 35.13 | 39.3 | CH2 | 1.51 | u | 2.15, 3.99 | C 1 |
| C 5 | 31.24 | 37.85 | CH | 2.15 | u | 1.42, 1.51 | C 19, C 1, C 9, C 3, C 7 |
| C 6 | 36.25 | 35.28 | CH2 | 1.35 | u | C 10, C 27, C 26 | |
| C 6 | 36.25 | 35.28 | CH2 | 1.42 | u | 1.64, 2.15, 3.79 | C 19, C 5, C 4, C 10, C 9, C 3 |
| C 7 | 67.35 | 69.57 | CH | 3.79 | u | 1.42, 1.48 | C 5, C 9 |
| C 8 | 39.81 | 40.66 | CH | 1.48 | u | 3.79 | C 19, C 15, C 11, C 20 |
| C 9 | 39.02 | 31.27 | CH | 1.68 | u | C 19, C 5, C 1, C 14, C 7, C 12 | |
| C 10 | 35.5 | 37.33 | C | ||||
| C 11 | 27.87 | 31.07 | CH2 | 1.57 | u | 3.95 | C 10, C 8, C 13 |
| C 11 | 27.87 | 31.07 | CH2 | 1.68 | u | ||
| C 12 | 72.61 | 70.62 | CH | 3.95 | u | 1.57 | C 18, C 11, C 9, C 14 |
| C 13 | 46.04 | 47.03 | C | ||||
| C 14 | 41.73 | 41.68 | CH | 1.94 | u | 1.12 | C 18, C 11, C 9, C 13, C 17, C 12 |
| C 15 | 22.72 | 26.12 | CH2 | 1.12 | u | 1.89, 1.94 | |
| C 15 | 22.72 | 26.12 | CH2 | 1.77 | u | C 8 | |
| C 16 | 27.31 | 27.34 | CH2 | 1.89 | u | 1.12, 1.85 | |
| C 16 | 27.31 | 27.34 | CH2 | 1.28 | u | C 13 | |
| C 17 | 47.08 | 47.04 | CH | 1.85 | u | 1.40, 1.89 | C 18, C 21, C 22, C 13 |
| C 18 | 11.45 | 18.13 | CH3 | 0.72 | S | C 14, C 13, C 17, C 12 | |
| C 19 | 8.89 | 20.63 | CH3 | 0.82 | S | C 1, C 10, C 9, C 8 | |
| C 20 | 35.76 | 35.6 | CH | 1.4 | u | 1.02, 1.11, 1.85 | C 23 |
| C 21 | 16.74 | 19.42 | CH3 | 1.02 | D | 1.4 | C 22, C 17 |
| C 22 | 35.98 | 36.61 | CH2 | 1.46 | u | C 21, C 24 | |
| C 22 | 35.98 | 36.61 | CH2 | 1.11 | u | 1.4 | |
| C 23 | 23.25 | 23.9 | CH2 | 1.48 | u | ||
| C 23 | 23.25 | 23.9 | CH2 | 1.31 | u | C 25 | |
| C 24 | 27.84 | 26.92 | CH2 | 1.35 | u | ||
| C 25 | 40.91 | 42.46 | CH | 1.8 | u | 3.60, 4.00 | C 23 |
| C 26 | 67.56 | 71.47 | CH2 | 4 | u | 1.8 | C 24, C 27 |
| C 26 | 67.56 | 71.47 | CH2 | 4.05 | u | ||
| C 27 | 61.73 | 63.11 | CH2 | 3.6 | u | 1.8 | |
| C 27 | 61.73 | 63.11 | CH2 | 3.56 | u | C 24, C 26 |
*Prediction of 13C chemical shifts was performed using the HOSE code based algorithm.
A slightly edited Molecular Connectivity Diagram (MCD) is presented in Figure 1.
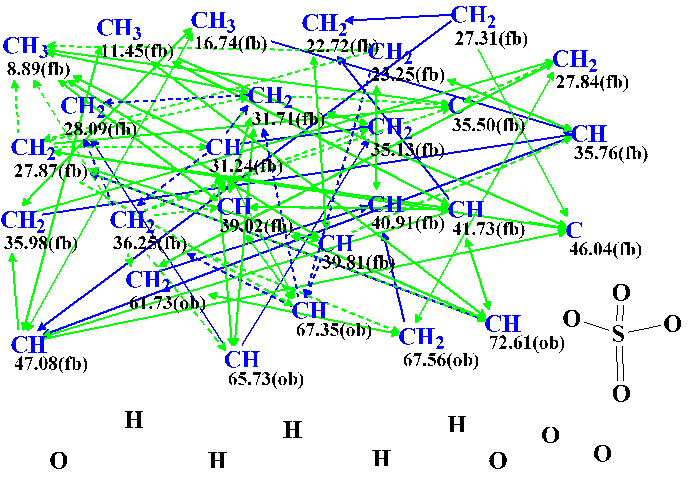
Figure 1. Slightly edited Molecular Connectivity Diagram.
MCD overview. The program automatically set the “fb” label for carbons whose 13C chemical shifts are assigned between 8.89 and 47.08 ppm, while the CH2 atom at 67.56 was marked by the “ob” label. All carbons of fb type can not have an oxygen in the first sphere of environment, but ob atoms must have an oxygen atom as a neighbor. Taking into account 13C and 1H chemical shifts of the carbons C 61.73, C 65.73, 67.35 and 72.61, these atoms were manually marked as ob.
Since the presence of the sulfate group was revealed by ESI-MS/MS the corresponding fragment was manually drawn in the MCD. Table 1 shows that there are three pairs of hydrogen atoms (marked by different colors) for which identical chemical shifts were assigned in the 1H NMR spectrum. This is the reason for the ambiguous HMBC and COSY correlations existence (the latter are denoted with dotted lines in MCD). The presence of ambiguous correlations is known to lead to an increase of the time of structure generation. To somehow reduce the generation time, the reliably determined multiplicities shown in Table 1 were used to set the total numbers of hydrogens attached to carbon atoms which are present in the first sphere of a given carbon environment.
Checking the MCD for consistency of the NMR data showed that there were contradictions. This means that there are correlations of nonstandard length (nJ, n>3) among HMBC and COSY data. Therefore Fuzzy Structure Generation was initiated with the options automatically determined by the program. Structure generation was accompanied with fast 13C chemical shift prediction and filtering of generated structures. Results: k = 10→5→4, tg= 9 m; the not empty structural file was saved when 4 connectivities have been varied during generation, while 375,970 connectivity combinations have been used.
Chemical shift predictions were carried out for all the structures obtained using the three empirical approaches implemented into ACD/Structure Elucidator – incremental, neural networks and HOSE code based. The output structural file was then ranked in increasing order of average deviations dA(13C) calculated by HOSE code approach. The ranked structures are presented in Figure 2.
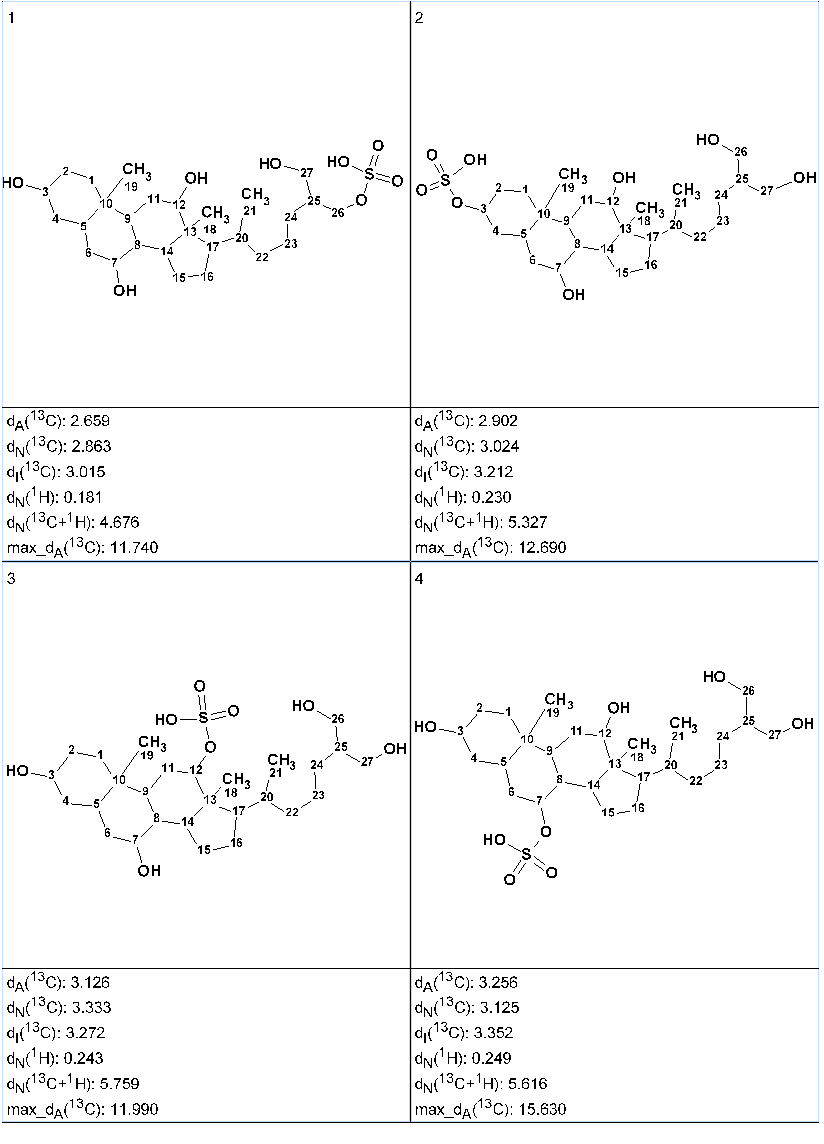
Figure 2. Ranked output structural file.
Figure 2 shows that the top ranked structure #1 is identical to the structure of 5α-cyprinol sulfate. The atom numbering displayed along with the structures shows that 13C and 1H chemical shift assignment automatically performed by the program coincided with that suggested by the authors in [1]. It is interesting to note that all four structures have the same carbon skeleton, while the difference between structures is in the position of the sulfate group, which “migrates” around the skeleton. In this situation, the structural information extracted from H2BC, 1D NOE, and 1D TOCSY spectra can not help to select a single plausible structure. It has been shown in [3,4] that DFT based chemical shift prediction could be used for additional confirmation.
The structure #1 together with 13C chemical shift assignment is shown below. The red arrows mark correlations of nonstandard length which were automatically detected by the program.
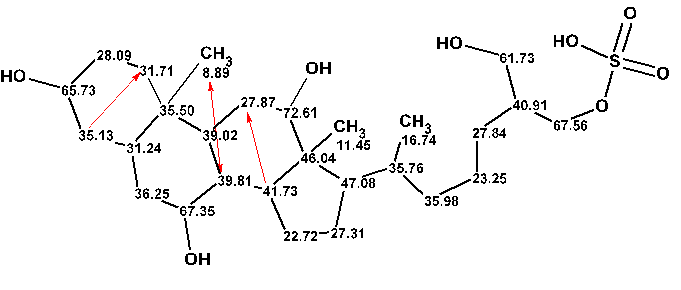
In conclusion the revision of the chemical shift assignment carried out by Hahn and coworkers was confirmed by the standard procedure of structure elucidation used in the ACD/Structure Elucidator system.
References
- M. Hahn, E. von Elert, L. Bigler, M. D. D. Hernández, N. E. Schloerer. 5α-Cyprinol sulfate: Complete NMR assignment and revision of earlier published data, including the submission of a computer‐readable assignment in NMReDATA format.
Magn Reson Chem., accepted 28 June 2018, DOI: 10.1002/mrc.4782 - M. Pupier, J.‐M. Nuzillard, J. Wist, N. E. Schlörer, S. Kuhn, M. Erdelyi, C. Steinbeck, A.J. Williams,C. Butts, T.D.W. Claridge, B. Mikhova, W. Robien, H. Dashti, H.R. Eghbalnia, C. Farès, C. Adam, P. Kessler, F. Moriaud, M. Elyashberg, D. Argyropoulos, M. Pérez, P.Giraudeau, R. R. Gil, P. Trevorrow, D. Jeannerat. (2018). NMReDATA, a standard to report the NMR assignment and parameters of organic compounds.
Magn Reson Chem., 56: 1–13. DOI: 10.1002/mrc.4737 - A. V. Buevich, M. E. Elyashberg. (2016). Synergistic combination of CASE algorithms and DFT chemical shift predictions: a powerful approach for structure elucidation, verification and revision.
J. Nat. Prod., 79(12): 3105–3116. - A.V. Buevich, M. E. Elyashberg. (2018). Towards unbiased and more versatile NMR-based structure elucidation: A powerful combination of CASE algorithms and DFT calculations.
Magn. Reson. Chem. 56: 493–504. DOI: 10.1002/mrc.4645


