December 1, 2016
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
Alistonitrine A
Natural products researchers, synthetic chemists and pharmacologists pay considerable attention to Monoterpene Indole Alkaloids (MIAs) because of their intricate structures and biological properties of interest.
MIAs are found in Alstonia scholaris R. Br. (Apocynaceae) which originates in southern China and several other South Asia locations. People in the area have been using the bark and leaves as traditional medicines. Zhu and co-workers [1] successfully isolated a new MIA, Alistonitrine A (1), which possesses an unprecedented caged monoterpene indole skeleton.
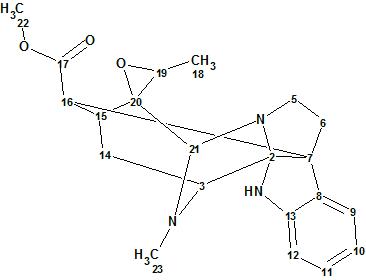
Figure 1. Structure of Alistronitine A
Alistonitrine A was isolated as a pale yellow powder. HR-MS data revealed a molecular formula of C21H25N3O3 with 11 degrees of unsaturation. Absorption bands at 1737 cm-1 and 1608 cm-1 in the FTIR spectrum indicate the presence of an ester carbonyl group and an aromatic ring. There were 21 carbon signals observed in the 13C NMR spectrum.
The NMR spectroscopic data used by Zhou [1] for the structure elucidation are shown in Table 1.
Table 1. Alistonitrine A. NMR spectroscopic data.
| Label | δC | δC* Calc | XHn | δH | COSY | H to C HMBC |
| C 2 | 101.7 | 96.31 | C | |||
| C 3 | 69.6 | 59.8 | CH | 3.03 | 2.42 | C 2, C 14, C 15, C 21, C 23 |
| C 5 | 44.7 | 51.97 | CH2 | 3.15 | 2.5 | C 2, C 6, C 7, C 21 |
| C 5 | 44.7 | 51.97 | CH2 | 2.86 | C 6, C 21 |
|
| C 6 | 43.6 | 36.35 | CH2 | 2.06 | C 2, C 16 |
|
| C 6 | 43.6 | 36.35 | CH2 | 2.5 | 3.15 | C 5, C 8, C 16 |
| C 7 | 53.5 | 60.11 | C | |||
| C 8 | 135.3 | 134.75 | C | |||
| C 9 | 122.5 | 123.7 | CH | 7.38 | 6.77 | C 7, C 11, C 13 |
| C 10 | 118.9 | 120.2 | CH | 6.77 | 7.06, 7.38 |
C 8, C 12 |
| C 11 | 127.9 | 128.46 | CH | 7.06 | 6.62, 6.77 |
C 9, C 13 |
| C 12 | 109.5 | 111.14 | CH | 6.62 | 7.06 | C 8, C 10 |
| C 13 | 146.4 | 149.07 | C | |||
| C 14 | 35.5 | 19.83 | CH2 | 2.42 | 1.78, 3.03 |
C 2, C 3, C 15, C 16 |
| C 14 | 35.5 | 19.83 | CH2 | 1.38 | C 3, C 15, C 16, C 20 |
|
| C 15 | 40.4 | 31.72 | CH | 1.78 | 2.42, 3.32 |
C 3, C 14, C 16, C 20, C 21 |
| C 16 | 51.4 | 46.24 | CH | 3.32 | 1.78 | C 2, C 6, C 7, C 8, C 14, C 15, C 17, C 20 |
| C 17 | 172.9 | 173.14 | C | |||
| C 18 | 13.5 | 15.32 | CH3 | 1.41 | 3.63 | C 19, C 20 |
| C 19 | 61.7 | 59.88 | CH | 3.63 | 1.41 | C 15, C 18, C 20 |
| C 20 | 61.3 | 62.67 | C | |||
| C 21 | 81.5 | 90.26 | CH | 3.51 | C 2, C 3, C 15, C 20, C 23 |
|
| C 22 | 51.6 | 51.78 | CH3 | 3.8 | C 17 | |
| C 23 | 42.5 | 33.71 | CH3 | 2.76 | C 3, C 21 |
|
| N 1 | NH | 4.11 |
* 13C chemical shift calculations were carried out using a HOSE code based approach [2]
These data were entered into the ACD/Structure Elucidator software and a Molecular Connectivity Diagram (MCD) was generated by the program (Figure 2).
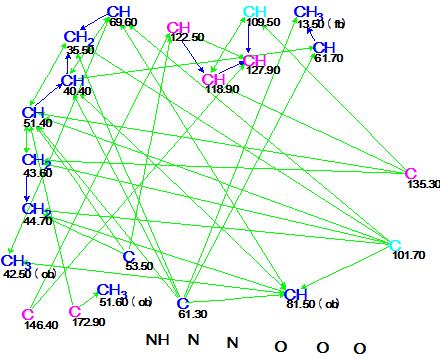
Figure 2. Alistonitrine A. Molecular connectivity diagram.
MCD overview. Hybridizations of all carbon atoms, except C 109.50 and C 101.70, were unambiguously determined by the program from the characteristic 13C and 1H chemical shifts. A hybridization of not sp (sp3 or sp2 ) was set for C 109.50 and C 101.70 (marked by light blue color). No MCD edits were made by the user. Since no non-standard correlations (nJCH,HH, n>3) were detected by the program in either the HMBC or COSY spectrum, the Strict Structure Generation was initiated which gave the following results: k = 28 →14, tg = 0.05 s. 13C chemical shift prediction was performed afterwards for all structures of the output file using the three empirical methods implemented into ACD/Structure Elucidator: HOSE code based, artificial neural networks and incremental [2]. The generated structures were ranked in increasing order of average chemical shift deviation values. The six top ranked structures are presented in Figure 3.
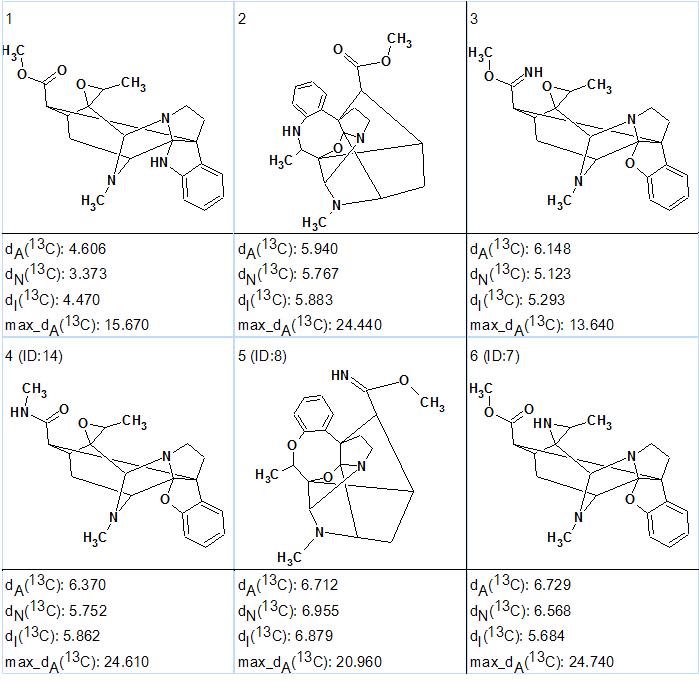
Figure 3. Alistonitrine A. The six top ranked structures of the output file.
Figure 3 shows that the highest ranked structure is identical to structure 1 of Alistonitrine determined in [1]. However the values of deviations corresponding to this structure are rather large which is expected for the unprecedented skeleton of the molecule under investigation. The validity of structure 1 is confirmed by the very large maximum deviations (20-25 ppm) calculated for the other candidate structures. Structure 3 should be eliminated due to the large average deviations but also because the characteristic stretching frequency of the C=N bond is usually observed between 1600-1660 cm-1[3], while a band at 1737 cm-1 characteristic for ester carbonyl was detected in the IR spectrum of the compound.
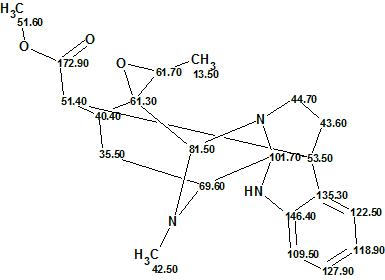
Figure 4. Structure 1 together with 13C chemical shift assignments.
Conclusions: The structure of a new complex natural product characterized by an unprecedented skeleton was automatically elucidated by ACD/Structure Elucidator. The structure was confirmed despite the relatively large values of average deviations found for the first ranked structure. It has also been shown [4] that in such a case a DFT 13C chemical shift prediction for structures 1 and 3 would be useful for further structure confirmation.
References
- G.-Y. Zhu, X.-J.Yao, L. Liu, L.-P. Bai, Z.-H. Jiang. (2014). Alistonitrine A, a Caged Monoterpene Indole Alkaloid from Alstonia scholaris. Org. Lett., 16:1080−1083.
- M.E. Elyashberg, A.J. Williams. (2015). Computer-based Structure Elucidation from Spectral Data (p. 454). Springer-Verlag Berlin, Heidelberg.
- D. Lin-Vien, N.B. Colthup, W.G. Fately, J.G. Grasselli. Infrared and Raman Characteristic Frequencies of Organic Molecules. Academic Press, San Diego, 1991.
- Buevich, A., Elyashberg, M. Synergistic combination of CASE algorithms and DFT chemical shift predictions: a new powerful approach for structure elucidation, verification and revision. SMASH-2016, September 11-14, 2016, La Jolla, USA.


