May 1, 2019
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
Cascarinoid A
Many plants of the Croton genus, found in the tropical and subtropical parts of the world, have been used in traditional medicine. Investigations of these plants have isolated several compounds from many different classes: Diterpenoids, polyphenols and alkaloids, to name a few. C. cascarilloides, a shrub from southern Asia, has been known to have interesting crotofolane diterpenoids. Gao et al [1] isolated, amongst others, Cascarinoid A (1), the first example of a crotofolane diterpenoid alkaloid. They elucidated the structure using spectroscopic data, single-crystal X-ray diffraction and ECD data. The planar structure obtained is shown in (1)
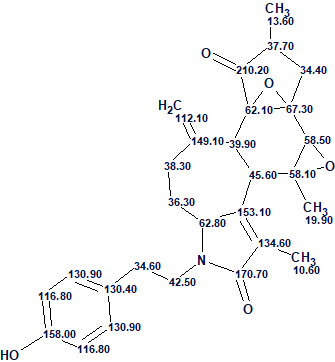
1
Cascarinoid A (1) was isolated as colorless crystals. The molecular formula C28H31NO5 was found based on (+)-HRESIMS, with a protonated molecular ion peak appearing at m/z 462.2285 [M +H]+ (calcd 462.2280) with 14 double-bond equivalents (DBEs). The IR spectrum indicated the presence of hydroxy (3431 cm−1), keto (1745 cm−1), and amide (1653 cm−1) groups.
The 1D and 2D NMR data from [1] are shown in Table 1. It is worth noting that only key 1H –13C HMBC correlations were available in [1].
Table 1. NMR spectroscopic data.
| C 1 | 210.200 | 210.640 | C | ||||
| C 2 | 37.700 | 42.640 | CH | 2.550 | u | 1.04, 1.72 |
|
| C 3 | 34.400 | 33.260 | CH2 | 2.730 | u | ||
| C 3 | 34.400 | 33.260 | CH2 | 1.720 | u | 2.55 | C 4 |
| C 4 | 62.100 | 67.580 | C | ||||
| C 5 | 58.500 | 62.780 | CH | 3.410 | s | C 4, C 14 |
|
| C 6 | 58.100 | 59.380 | C | ||||
| C 7 | 45.600 | 47.630 | CH | 3.290 | d | 2.82 | C 8 |
| C 8 | 153.100 | 159.480 | C | ||||
| C 9 | 62.800 | 63.970 | CH | 3.970 | u | 2.47 | C 8 |
| C 10 | 36.300 | 29.330 | CH2 | 2.470 | u | 2.32, 3.97 |
|
| C 10 | 36.300 | 29.330 | CH2 | 0.770 | u | ||
| C 11 | 38.300 | 35.730 | CH2 | 2.320 | u | 2.47 | |
| C 11 | 38.300 | 35.730 | CH2 | 2.570 | u | ||
| C 12 | 149.100 | 147.270 | C | ||||
| C 13 | 39.900 | 47.400 | CH | 2.820 | d | 3.29 | C 11, C 14, C 12, C 1 |
| C 14 | 67.300 | 67.590 | C | ||||
| C 15 | 134.600 | 128.280 | C | ||||
| C 16 | 170.700 | 171.840 | C | ||||
| C 17 | 10.600 | 8.400 | CH3 | 2.030 | s | C 15, C 8, C 16 |
|
| C 18 | 112.100 | 113.680 | CH2 | 4.970 | u | ||
| C 18 | 112.100 | 113.680 | CH2 | 5.180 | u | C 11, C 12 |
|
| C 19 | 13.600 | 15.640 | CH3 | 1.040 | d | 2.55 | C 1 |
| C 20 | 19.900 | 20.150 | CH3 | 1.050 | s | C 7, C 6, C 5 |
|
| C 21 | 42.500 | 42.900 | CH2 | 3.380 | u | ||
| C 21 | 42.500 | 42.900 | CH2 | 4.340 | u | 2.88 | C 9, C 16 |
| C 22 | 34.600 | 34.440 | CH2 | 2.880 | u | 4.34 | C 23, C 24 |
| C 22 | 34.600 | 34.440 | CH2 | 3.120 | u | ||
| C 23 | 130.400 | 131.290 | C | ||||
| C 24 | 130.900 | 130.780 | CH | 7.260 | d | 7.15 | |
| C 25 | 116.800 | 116.200 | CH | 7.150 | d | 7.26 | C 23, C 26 |
| C 26 | 158.000 | 156.800 | C |
*Calculation of 13C chemical shifts was performed using the HOSE code-based approach.
The molecular formula of compound 1 and the tabulated spectroscopic data were entered into ACD/Structure Elucidator. Figure 1 shows the molecular connectivity diagram (MCD) created by the program.
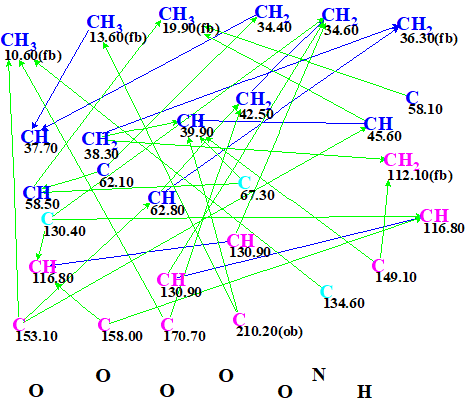
Figure 1. Molecular connectivity diagram (MCD)
MCD overview. Only six carbon atoms of the 28 were labeled according to the presence (“ob“, obligatory) or absence (“fb“, forbidden) of a heteroatom in the first sphere of the corresponding carbon atom environment. Three quaternary carbons (C 67.3, C 130.4 and C 134.6) are colored in light blue, indicating an ambiguous hybridization (sp3 or sp2, but not sp). The large number of carbons without any ob/fb labels is because of the presence of a nitrogen atom: under this condition selection of a definite label becomes problematic.
No edits to the MCD were made and the structure generation, accompanied with 13C chemical shift prediction and spectral filtering, was initiated. Results: k=612→5→4, tg = 1h 16 m. The relatively long time of structure generation could be attributed to the atom properties ambiguity in the MCD and to the lack of HMBC correlations.
The output structural file, ranked in ascending order of 13C chemical shift average deviation, is presented in Figure 2.
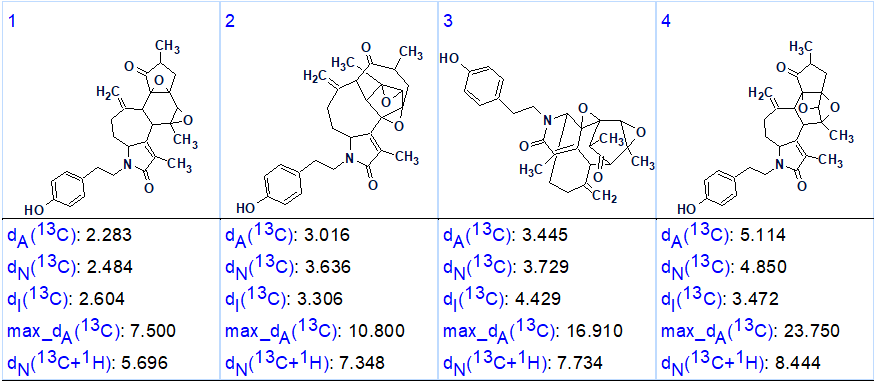
Figure 2. Ranked output structural file.
We see that the average deviations calculated by all three methods common for ACD/SE [2] are minimal for structure #1 which coincides with the structure of cascarinoid A determined by authors [1] and confirmed by X-ray crystallography. The structure together with the automatically assigned 13C chemical shifts is shown below:
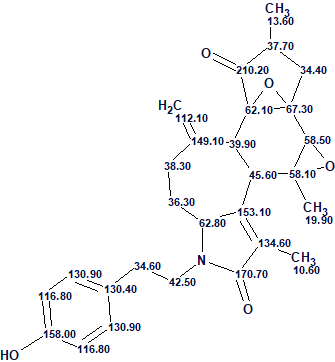
This case can be used to see how the results of structure generation will depend on additional constraints imposed on the initial data which are presented in the MCD.
The following kinds of additional information can be considered:
-
- Signal multiplicities determined in 1H NMR (see column M in Table 1). The values of hydrogen atoms existing in the first sphere of a carbon atom environment can be introduced in the dialog window “Edit Atom Properties” in the MCD [2].
- Suggestion that carbon C 170.7 is bonded to a heteroatom (O or N). The corresponding fragment of MCD is shown below
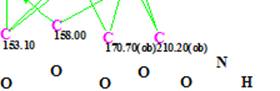
-
- Suggestion that carbon C 170.7 is a carbonyl. The corresponding fragment of MCD is shown below
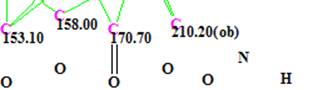
The different combinations of the mentioned constraints and corresponding results of structure generation are summarized in Table 2.
Table 2. Combinations of constraints and results of structure generation.
| # | C(170) | C(170.7) ob | C(170.7) =O | M | tg, min | k |
| 1 | – | – | – | – | 76 | 612→5 |
| 2 | – | – | – | + | 29 | 557→5 |
| 3 | – | + | – | – | 24.5 | 423→5 |
| 4 | – | + | – | + | 9.5 | 368→5 |
| 5 | – | – | + | – | 2 | 423→5 |
| 6 | – | – | + | + | 1 | 368→5 |
We see that employing multiplicities in 1H NMR (combination 2) reduces the time of structure generation by a factor of two. Supplying the carbon C 170.7 with a label “ob” leads to almost the same results (combination 3), however the tg value drops down to 9.5 min if setting label “ob” is combined with the usage of multiplicities (combination 4). All mentioned suggestions are not risky as we allow the carbon C 170.7 being connected to either O or N (fragments =C-O, C=O, C=N, =C-N are allowed). But if we suggest that the carbon C 170.7 belongs to a carbonyl group (according to IR two carbonyls exist in the structure, and one of them, C 210.2, has been already detected by the program) the time reduces to 2 min (combination 5). If, in addition, multiplicities are also taken into account (combination 6), the tg goes down to 1 min.
This “little investigation” demonstrates how the results of structure generation are sensitive to any additional constraints introduced by the user. The user should realize that he or she takes the responsibility for introducing mistaken assumption (axioms) in the game. Therefore, for the sake of safety, it is recommended to use only such additional information for which there is a high level of confidence.
References
- X.-H. Gao, Y.-S. Xu, Y.-Y. Fan, L.-S. Gan, J.-P. Zuo, J-M. Yue. (2018). Cascarinoids A−C, a Class of Diterpenoid Alkaloids with Unpredicted Conformations from Croton cascarilloides. Org. Lett., 20 (1): 228–231, DOI: 10.1021/acs.orglett.7b03592
- M. E. Elyashberg, A. J. Williams. (2015). Computer-based Structure Elucidation from Spectral Data. The Art of Solving Problems. Springer.


