November 1, 2016
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
Euphorikanin A
Euphorbia kansui is a plant commonly found in northern China. Its roots are used in Traditional Chinese medicine to treat various diseases. Previous studies have shown that the plant contains diterpenoids, triterpenes, and phenolic derivatives,1 and the major bioactive components were found to be the diterpenoids.2,3 Fei and co-workers4 isolated Euphorikanin A (1), a structurally novel diterpenoid lactone with an unprecedented carbon skeleton featuring a unique tetradecahydrobenzo[cd]-cyclopropa[f ]azulene. Its structure was determined by spectroscopic methods and confirmed by single crystal X-ray analysis.
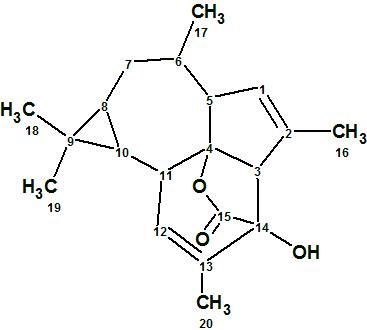
1
Euphorikanin A was crystalized from a petroleum ether—acetone 5:1 solution in the form of needle crystals. The crystals exhibited optical activity when dissolved in CHCl3 ([α]D25 +70). High Resolution positive ion MS indicated a molecular formula of C20H26O3, with the [M + H]+ found at m/z of 315.1955, vs. a calculated m/z of 315.1955. This was in agreement with 1H and 13C NMR spectroscopic data. Characteristic bands for an ester carbonyl (1756 cm-1) and hydroxyl groups (3442 cm-1) were observed in the IR spectrum.
To elucidate the structure of Euphorikanin A, the authors4 used HSQC, COSY, and HMBC along with 1D NMR spectra. The COSY data were omitted when ACD/Structure Elucidator was challenged with this problem. The spectroscopic information entered into the program is summarized in Table 1, while the automatically generated Molecular Connectivity Diagram (MCD) corresponding to these data is shown in Figure 1.
Table 1. Euphorikanin A. NMR data used.
| Label | δC | δC calc* | XHn | δH | C HMBC |
| C 1 | 130.6 | 123.89 | CH | 5.53 | C 3, C 4 |
| C 2 | 137.3 | 144.07 | C | ||
| C 3 | 59.6 | 59.21 | CH | 3.09 | C 2, C 4, C 13, C 14, C 15 |
| C 4 | 97.3 | 91.57 | C | ||
| C 5 | 54.5 | 55.29 | CH | 2.71 | C 3, C 4 |
| C 6 | 31.3 | 39.69 | CH | 1.34 | C 4, C 5, C 8, C 17 |
| C 7 | 32.7 | 32.33 | CH2 | 1.79 | C 6, C 8, C 9, C 10, C 17 |
| C 7 | 32.7 | 32.33 | CH2 | 1.68 | |
| C 8 | 20.8 | 22.55 | CH | 0.68 | C 6 |
| C 9 | 18.8 | 22.12 | C | ||
| C 10 | 26.5 | 26.12 | CH | 0.81 | C 8, C 12, C 18, C 19 |
| C 11 | 39.1 | 42.98 | CH | 2.34 | |
| C 12 | 126.1 | 128.97 | CH | 5.4 | C 4, C 14 |
| C 13 | 139.5 | 136.63 | C | ||
| C 14 | 80.4 | 80.07 | C | ||
| C 15 | 177 | 169.69 | C | ||
| C 16 | 16.2 | 17.49 | CH3 | 1.84 | C 1, C 2, C 3 |
| C 17 | 20.9 | 18.38 | CH3 | 0.89 | C 5, C 7 |
| C 18 | 28.6 | 26.5 | CH3 | 1.05 | C 8, C 10 |
| C 19 | 15.3 | 13.5 | CH3 | 1 | C 9, C 18 |
| C 20 | 16.6 | 16.26 | CH3 | 1.81 | C 12, C 13, C 14 |
| O 1 | OH | 2.72 | C 3, C 14, C 15 |
* 13C chemical shifts were calculated using HOSE codes based approach.
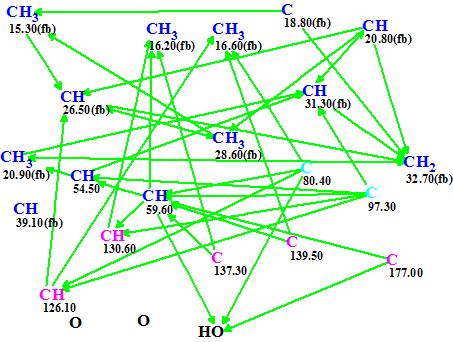
Figure 1. Euphorikanin A. Molecular Connectivity Diagram.
MCD overview. Carbon atoms colored blue are sp3-hybridized, and the fb label is used to indicate that heteroatoms cannot be connected to them. Carbon atoms colored pink are sp2-hybridized. Two carbon atoms, C 80.40 and 97.30, are colored in light blue which means that their hybridization is sp3 or sp2, but not sp. Note that the CH carbon at 39.10 ppm has no connectivity to other atoms that were revealed by NMR.
There were no manual edits of the MCD required. Strict Structure Generation accompanied with 13C chemical shift prediction and spectral structure filtering was performed and the following results were obtained: k=4→2, tg=0.09 s. Two possible structures, ranked in order of average 13C chemical shift deviation increase, were generated and are presented in Figure 2.
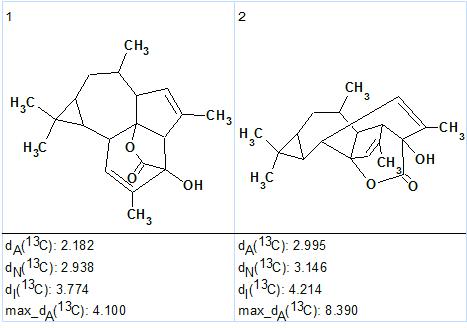
Figure 2. Euphorikanin A. Top 2 Ranked structures generated.
The first structure coincides with that of Euphorikanin A (1) previously reported.4 The elucidated structure and its automatically assigned 13C chemical shifts are shown below.
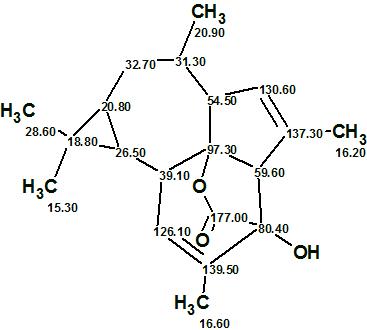
1
We see that the structure of Euphorikanin A, which possesses an unprecedented carbon skeleton, was elucidated with the aid of ACD/Structure Elucidator fully and automatically in almost no time (0.09 s). It is important to note that COSY data were not used in this study.
References
- Shi, Q. W., Su, X. H., Kiyota, H. (2008). Chemical and Pharmacological Research of the Plants in Genus Euphorbia. Chem. Rev., 108(10):4295−4327.
- Vasas, A., Hohmann, J. (2014). Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008–2012). Chem. Rev., 114(17):8579−8612.
- Vasas, A.; Rédei, D.; Csupor, D.; Molnár, J.; Hohmann, J. (2012). Diterpenes from European Euphorbia Species Serving as Prototypes for Natural-Product-Based Drug Discovery. Eur. J. Org. Chem., 2012(27):5115−5130.
- Fei, D.-Q.; Dong, L.-L.; Qi, F.-M.; Fan, G.-X.; Li, H.-H.; Li, Z.-Y.; Zhang, Z.-X. (2016). Euphorikanin A, a Diterpenoid Lactone with a Fused 5/6/7/3 Ring System from Euphorbia kansui.
Org. Lett., 18(12):2844–2847.


