February 1, 2022
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
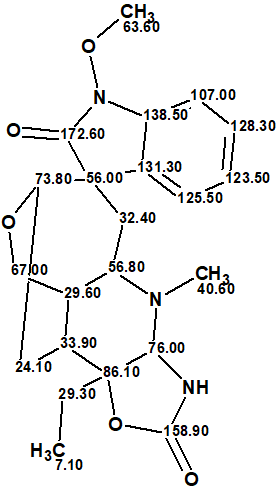
Gelstriamine A
The condensation of secologanin and tryptophan gives rise to the Monoterpene Indole Alacloids (MIAs). The unique feature in their structure is that they have 2 nitrogen atoms. MIAs are know to have good properties as drugs and hence have been studied extensively by scientists. A potential source of MIAs is Gelsenium elegans (Gardn. & Champ.) known as “Duanchangcao” or “Dechayao”, amongst others. It has been used in China as an analgesic and various MIAs have been isolated from its leaves, fruits, and roots.
Jin et al [1] studied the novel analgesics used in traditional Chinese medicine and as part of this they isolated and elucidated the structure of a novel triamine monoterpene indole alkaloid, gelstriamine A (1) which possessed a new skeleton.
1
Gelstriamine A possesses a cagelike, 6/5/7/6/6/5 heterohexacyclic scaffold bearing a unique hexahydrooxazolo[4,5-b]pyridin-2(3H)-one motif and represents the first natural product containing such a unit.
Gelstriamine A (1) was isolated as a colorless oil, and the molecular formula C22H27N3O5 was assigned by the 13C NMR data and a HR-ESI-MS ion at m/z 414.2009 [M + H]+ (calcd for C22H28N3O5, 414.2029), indicating 11 degrees of unsaturation.
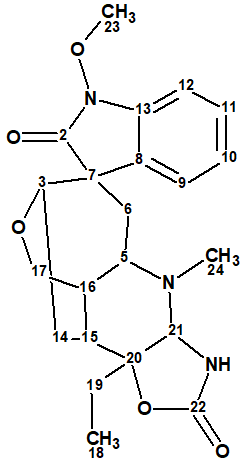
1D and 2D NMR spectroscopic data (see Table 1) which served the basis for gelstriamine A structure elucidation were used by us for challenging ACD/Structure Elucidator (ACD/SE).
Table 1. 1D and 2D NMR spectroscopic data.
| Label | δC | δC calc HOSE | XHn | δH | COSY | H to C HMBC |
| C 2 | 172.6 | 174.13 | C | |||
| C 3 | 73.8 | 71.65 | CH | 3.6 | 2.04 | C 17, C 8 |
| C 5 | 56.8 | 57.31 | CH | 3.12 | 2.14, 2.56 | |
| C 6 | 32.4 | 31.2 | CH2 | 2.26 | ||
| C 6 | 32.4 | 31.2 | CH2 | 2.14 | 3.12 | C 3, C 8, C 2 |
| C 7 | 56 | 54.75 | C | |||
| C 8 | 131.3 | 130.99 | C | |||
| C 9 | 125.5 | 125.51 | CH | 7.37 | 7.11 | C 7, C 13 |
| C 10 | 123.5 | 123.36 | CH | 7.11 | 7.28, 7.37 | |
| C 11 | 128.3 | 128.15 | CH | 7.28 | 6.93, 7.11 | |
| C 12 | 107 | 107.01 | CH | 6.93 | 7.28 | C 8 |
| C 13 | 138.5 | 138.65 | C | |||
| C 14 | 24.1 | 28.89 | CH2 | 2.96 | ||
| C 14 | 24.1 | 28.89 | CH2 | 2.04 | 2.11, 3.60 | C 20 |
| C 15 | 33.9 | 34.79 | CH | 2.11 | 2.04, 2.56 | |
| C 16 | 29.6 | 40.88 | CH | 2.56 | 2.11, 3.12, 4.03 | |
| C 17 | 67 | 65.42 | CH2 | 4.36 | ||
| C 17 | 67 | 65.42 | CH2 | 4.03 | 2.56 | |
| C 18 | 7.1 | 7.82 | CH3 | 1.05 | 1.93 | |
| C 19 | 29.3 | 24.13 | CH2 | 1.83 | ||
| C 19 | 29.3 | 24.13 | CH2 | 1.93 | 1.05 | C 15, C 21 |
| C 20 | 86.1 | 89.81 | C | |||
| C 21 | 76 | 76.58 | CH | 4.62 | C 15, C 22 | |
| C 22 | 158.9 | 160.43 | C | |||
| C 23 | 63.6 | 63.28 | CH3 | 3.98 | ||
| C 24 | 40.6 | 37.72 | CH3 | 2.45 | C 5, C 21 | |
| N 1 | NH | 6.45 |
The data were entered into ACD/SE and a Molecular Connectivity Diagram (MCD) was created by the program (Figure 1).
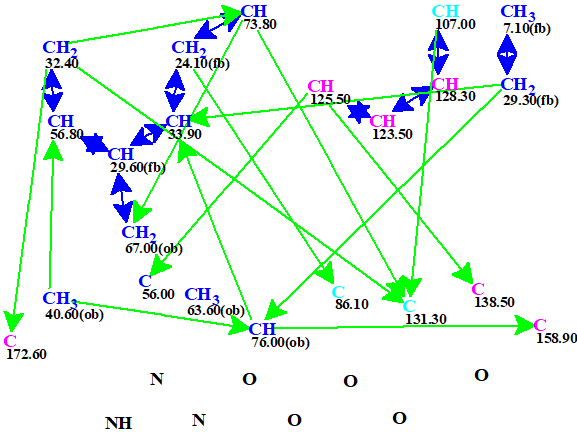
Figure 1. Automatically created MCD. Hybridizations of carbon atoms are marked by the corresponding colors: sp2 – violet, sp3 – blue, not sp – light blue. Labels “ob” and “fb” are set by the program to carbon atoms for which neighboring with heteroatom is either obligatory (ob) or forbidden (fb).
No edits were made to the MCD and standard structure generation was initiated. This process was accompanied by 13C chemical shift prediction using the incremental algorithm. The average deviations of calculated values from experimental ones (dI) were calculated and structures for which dI>6 ppm were rejected. Results: 42235 → (spectral filtering) → 778 → (removal of duplicates) → 778, tg = 1m 28 s.
Next 13C chemical shift prediction was carried out using the HOSE code-based and neural networks methods. The average deviations of 13C chemical shifts determined by these methods are denoted as dA and dN correspondingly. Finally, the output file was ranked in increasing order of dA values. Eight top ranked structures of the ranked file are shown in Figure 2.
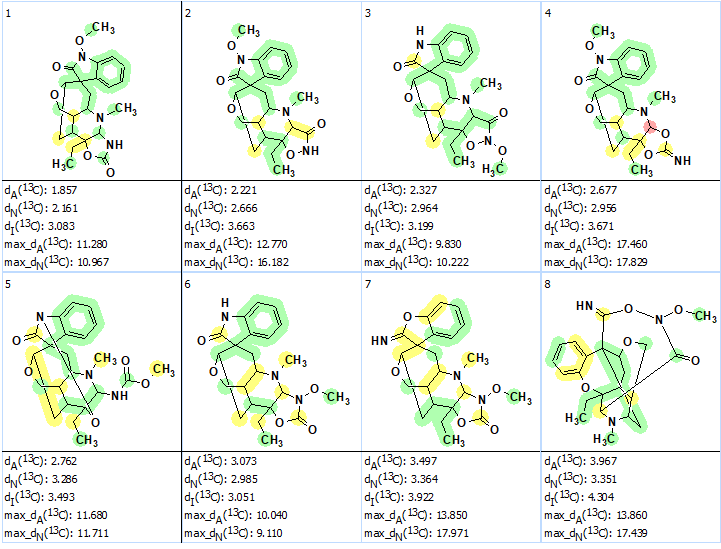
Figure 2. The eight top ranked structures of the output file. Each atom is colored to mark the difference between its experimental and calculated 13C chemical shifts. The green color represents a difference between 0 to 3 ppm, yellow was between 3 and 15 ppm and red was >15 ppm.
We see that the ranking procedure selected the best structure which is identical to the structure of gelstriamine A determined by the authors [1]. The DP4 probabilities were calculated for structures #1 – #6 afterwards, which confirmed that the best structure was the same as the one reported in [1] (see Figure 3).
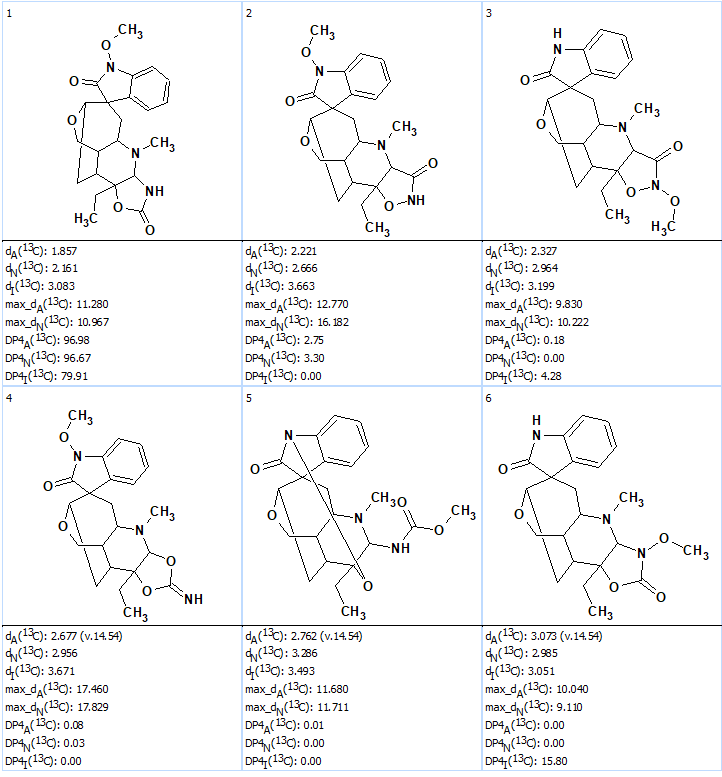
Figure 3. DP4 probabilities calculated for the first six structures of the ranked output file.
Consequently, the structure of an unknown characterized by an unprecedented skeleton was unambiguously elucidated by ACD/SE in fully automatic mode without any user intervention in 1.5 min. The structure of gelstriamine A together with the automatically assigned 13C chemical shifts is shown below.