May 1, 2014
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
Mandelalide A
Sikorska et al [1] isolated and investigated the unusual polyketide macrolides Mandelalides A-D which were isolated from a new species of Lissoclinum ascidian. Their planar structures were elucidated from sub-milligram samples by comprehensive analysis of 1D and 2D NMR data, and supported by mass spectrometry. Here we will describe the elucidation of Mandelalide A (1) using ACD/Structure Elucidator Suite.
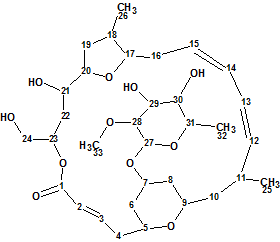
1
The HR-ESI-MS data for Mandelalide A gave a pseudo-molecular ion [M + Na]+ at m/z 647.3394, which is consistent with a molecular formula of C33H52O11, and implies 8 degrees of unsaturation.
For structure elucidation, the authors [1] used 1D NMR spectra in combination with HSQC, HMBC, COSY, TOCSY and ROESY data acquired at 700 (1H) and 175 (13C) MHz on a 5 mm inverse cryogenic probe. Because the molecular formula contains a large number of hydrogen atoms and produces a rich HMBC spectrum, we input only 1D, HSQC and HMBC data (Table 1) into the software.
Table 1. NMR spectroscopic data for Mandelalide A.
| Label | δC | δC calc. | CHn | δH | M/J | HMBC |
| C 1 | 167.4 | 166.58 | C | |||
| C 2 | 123.1 | 124.78 | CH | 6.01 | u | C 1, C 4 |
| C 3 | 147.1 | 146.47 | CH | 6.97 | u | C 1, C 2, C 4, C 5 |
| C 4 | 38.8 | 38.28 | CH2 | 2.36 | u | C 5, C 6, C 2, C 3 |
| C 4 | 38.8 | 38.28 | CH2 | 2.39 | u | |
| C 5 | 73.9 | 69.16 | CH | 3.36 | u | C 3, C 4 |
| C 6 | 37.6 | 38.46 | CH2 | 1.2 | u | C 4, C 5, C 8 |
| C 6 | 37.6 | 38.46 | CH2 | 2.02 | u | C 8, C 7 |
| C 7 | 73.1 | 67.97 | CH | 3.82 | u | C 27, C 9, C 8 |
| C 8 | 39.7 | 39.45 | CH2 | 1.87 | u | |
| C 8 | 39.7 | 39.45 | CH2 | 1.22 | u | C 6, C 9, C 7, C 10 |
| C 9 | 72.5 | 67.6 | CH | 3.32 | u | C 7, C 5 |
| C 10 | 43.1 | 42.64 | CH2 | 1.21 | u | C 11, C 25, C 12 |
| C 10 | 43.1 | 42.64 | CH2 | 1.51 | u | C 11, C 8, C 9, C 25, C 12 |
| C 11 | 34.2 | 33.89 | CH | 2.37 | u | C 12, C 10, C 25 |
| C 12 | 141.5 | 139.08 | CH | 5.45 | u | C 14, C 11, C 10, C 25 |
| C 13 | 123.9 | 131.19 | CH | 6.28 | u | C 15, C 14, C 10, C 11 |
| C 14 | 131.3 | 132.11 | CH | 6.05 | u | C 12, C 16, C 17, C 13 |
| C 15 | 126.9 | 132.1 | CH | 5.28 | u | C 13, C 17, C 16 |
| C 16 | 31.1 | 38.96 | CH2 | 2.28 | u | C 14, C 17, C 15, C 18 |
| C 16 | 31.1 | 38.96 | CH2 | 1.88 | u | C 14, C 17, C 15 |
| C 17 | 81 | 85.03 | CH | 3.98 | u | C 20, C 15, C 19 |
| C 18 | 37.3 | 34.79 | CH | 2.52 | u | C 16, C 19, C 17, C 26 |
| C 19 | 36.8 | 37.25 | CH2 | 2.01 | u | C 18, C 17 |
| C 19 | 36.8 | 37.25 | CH2 | 1.17 | u | C 21, C 18, C 20, C 26 |
| C 20 | 83.2 | 82.17 | CH | 3.63 | u | C 22, C 21 |
| C 21 | 73 | 72.58 | CH | 3.42 | u | C 22, C 20, C 23 |
| C 22 | 34.1 | 35.08 | CH2 | 1.76 | u | C 24, C 23 |
| C 22 | 34.1 | 35.08 | CH2 | 1.46 | u | C 20, C 21 |
| C 23 | 72.3 | 70.17 | CH | 5.23 | u | C 1 |
| C 24 | 66.1 | 64.67 | CH2 | 3.81 | u | |
| C 24 | 66.1 | 64.67 | CH2 | 3.61 | u | C 22, C 23 |
| C 25 | 18.3 | 17.5 | CH3 | 0.85 | u | C 10, C 11, C 12 |
| C 26 | 14.5 | 15.36 | CH3 | 1.03 | u | C 18, C 17, C 19 |
| C 27 | 94.2 | 95.8 | CH | 5.02 | u | C 29, C 28, C 31, C 7 |
| C 28 | 80.8 | 85.58 | CH | 3.4 | u | C 30, C 29, C 33 |
| C 29 | 71.7 | 77.98 | CH | 3.68 | u | |
| C 30 | 74.3 | 76.59 | CH | 3.34 | u | C 29, C 32, C 31 |
| C 31 | 68.1 | 70.74 | CH | 3.62 | u | C 29, C 30 |
| C 32 | 17.7 | 15.91 | CH3 | 1.27 | u | C 30, C 31 |
| C 33 | 59.1 | 60.64 | CH3 | 3.45 | u | C 28 |
The Molecular Connectivity Diagram (MCD, slightly edited) is presented in Figure 1.
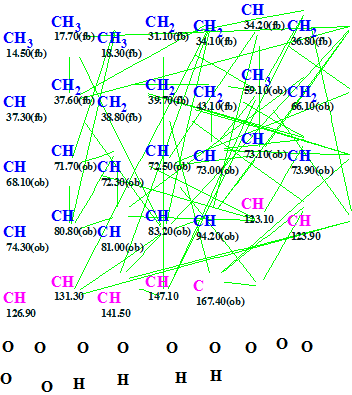
Figure 1. HMBC Molecular Connectivity Diagram for Mandelalide A.
MCD overview. According to the characteristics of the 13C and 1H chemical shifts (see Table 1) carbon atoms C(14.50)–C(43.10) were supplied with the property sp3/fb (forbidden), while carbons C(59.10)–C (94.2) were assigned as sp3/ob (obligatory). Carbon C(167.4) was given hybridization sp2/ob. Figure 1 shows that the unknown molecule must contain four hydroxyl groups, and that seven oxygen atoms should be connected to carbons only.
Checking the MCD with the software gave the message “The minimum number of non-standard connectivities is 2”. Therefore, Fuzzy Structure Generation (FSG) was initiated with automatic option selection, which gave the following results: k = 1, tg = 3s. Two of the 63 correlations were extended during structure generation, and only 42 of the 1953 possible connectivity combinations were used (~2%).
The output structure 1a coincided with the structure of mandelalide A, and displayed the following deviation values: dA=2.4, dN = 1.78, dI = 1.56 ppm. Automatic chemical shift assignment provided the same outcome as originally suggested by authors [1]. Red arrows denote two nonstandard HMBC correlations.
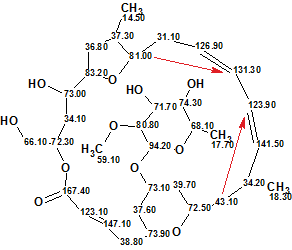
1a
Therefore the structure of a new natural product—an unusual polyketide macrolide—was instantly and unambiguously elucidated using only HMBC correlations by standard practices using ACD/Structure Elucidator Suite. Two nonstandard connectivities and their lengths were identified by the system automatically. Multiplicities in the 1H NMR spectrum were not used. This example succinctly demonstrates the power of applying a CASE system such as Structure Elucidator Suite to determine the structures of novel unknown compounds.
References
1. J. Sikorska, A.M. Hau, C. Anklin, S. Parker-Nance, M. T. Davies-Coleman, J. E. Ishmael, K. L. McPhail (2012). Mandelalides A-D, Cytotoxic Macrolides from a New Lissoclinum Species of South African Tunicate. J. Org. Chem., 77(14), 6066–6075.


