July 1, 2019
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
Orbicularisine
Mollusks are the largest marine phylum, comprising about 23% of all the named marine organisms. Despite this not more than 1000 have been studied chemically, which lead to the isolation and characterization of about 1000 secondary metabolites. It is thus easy to understand why marine mollusks are interesting for natural products studies aimed at the isolation of new metabolites. This becomes even more important if we take into account that some of these metabolites have therapeutic value and, in fact, Adcetris, also known as brentuximab vedotin, is a marine drug that has successfully entered the market.
Goudou at al [1] described the isolation of a novel metabolite, orbicularisine (1), from the tropical bivalve Codakia orbicularis living in seagrass beds.
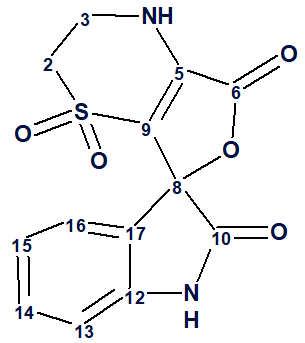
1
Even though this mollusk has been studied extensively as a biological model for bacterial symbiosis, no chemical studies had been performed so far.
The molecular formula of compound 1 was found to be C13H10N2O5S by HR-ESI-MS, which showed a protonated molecule at m/z 307.0391 [M + H]+, correct for 10 degrees of unsaturation. The structure of compound 1 was determined from MS, IR and NMR spectra (1H, 13C, COSY, 1H-13C HMBC, 1H-15N HSQC). The NMR data of orbicularisine (Table 1) were used for challenging ACD/Structure Elucidator.
Table 1. Spectroscopic data of compound 1
| Label | δC | δC calca | XHn | δH | COSY | H to C HMBC |
| C 2 | 48.100 | 49.630 | CH2 | 3.370 | ||
| C 2 | 48.100 | 49.630 | CH2 | 3.010 | 3.89 | C 8, C 9 |
| C 3 | 40.800 | 39.780 | CH2 | 3.680 | ||
| C 3 | 40.800 | 39.780 | CH2 | 3.890 | 3.01, 8.47 | C 5 |
| C 5 | 138.300 | 133.240 | C | |||
| C 6 | 165.100 | 159.360 | C | |||
| C 8 | 82.200 | 85.310 | C | |||
| C 9 | 111.200 | 109.580 | C | |||
| C 10 | 170.900 | 173.440 | C | |||
| C 12 | 142.800 | 139.860 | C | |||
| C 13 | 110.800 | 111.560 | CH | 6.910 | 7.36 | |
| C 14 | 132.100 | 130.730 | CH | 7.360 | 6.91, 7.02 | C 12 |
| C 15 | 122.600 | 125.310 | CH | 7.020 | 7.36, 7.39 | C 17 |
| C 16 | 126.600 | 124.570 | CH | 7.390 | 7.02 | C 8 |
| C 17 | 122.400 | 125.020 | C | |||
| N 1 | NH | 8.470 | 3.89 | C 9, C 6 | ||
| N 2 | NH | 10.960 | C 8, C 17, C 12, C 10 |
aChemical shift calculations were performed using the HOSE code based algorithm.
Based on the comparison of NMR spectra with those published in literature, the authors suggested the presence of an SO2 group in the molecule, which was confirmed by the IR spectrum (Figure 1), where a characteristic strong band from ns (SO2) at 1130 cm-1 is observed (the band of nas (SO2) at 1300 cm-1 is of medium intensity).
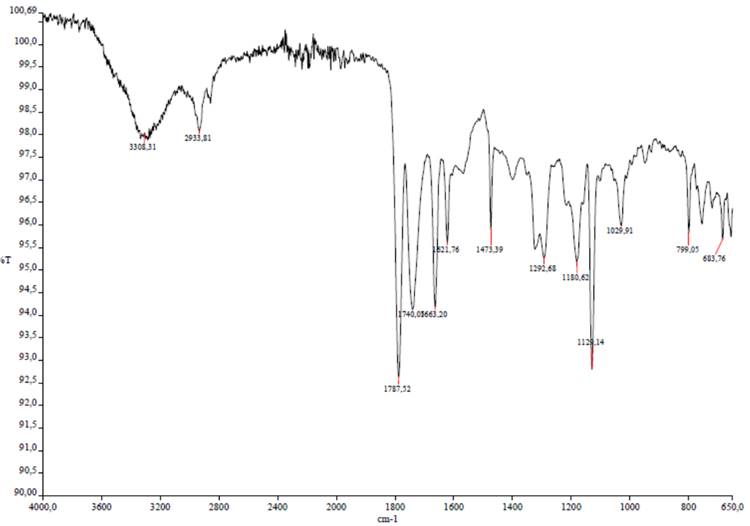
Figure 1. IR spectrum of compound 1.
Two carbonyl bands at 1785 and 1740 cm-1 indicate the presence of CO groups in strained five-membered rings. With this in mind, the slightly edited Molecular Connectivity Diagram (MCD) presented in Figure 2 was created.
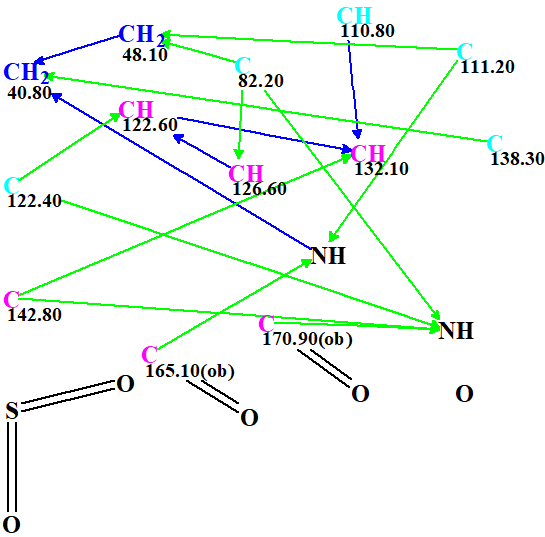
Figure 2. Molecular connectivity diagram.
The edits consisted of manually drawing an SO2 group and two carbonyls. The MCD was checked for contradictions by the program and it passed all tests. This means that no COSY or HMBC correlations of non-standard length (Non Standard Correlations, NSC) were detected, therefore Strict Structure Generation was initiated with the Options shown in Figure 3.
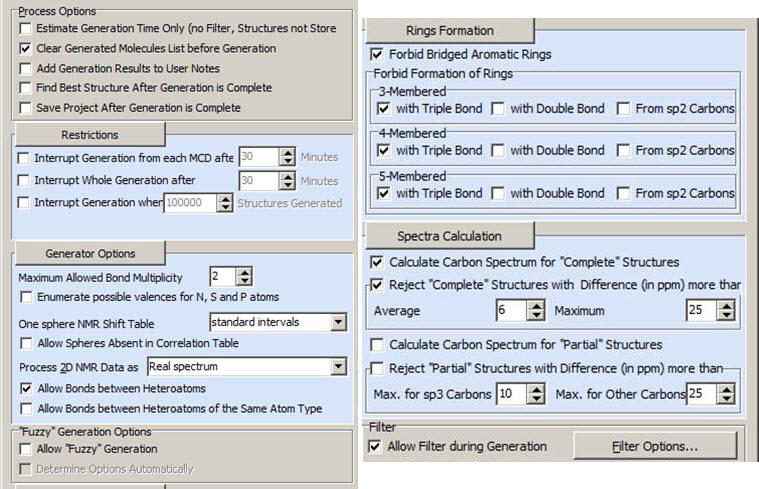
Figure 3. Options of Strict Structure Generation.
Results of structure generation: k = 173→1, tg = 0.3 s. The single generated structure is shown in Figure 4.
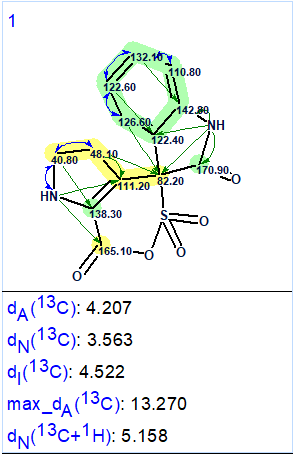
Figure 4. Structure produced by the program as a result of Strict Structure Generation
We see that this structure is not the same as 1 also that all calculated average deviations are too large. The upper part of the molecule, colored in green, has good agreement between the experimental and the predicted 13C chemical shift values. The lower part, however, colored in yellow, does not have such a good agreement. Even though there are a few reasons why this could happen the most probable reason is the presence of NSCs in the 2D NMR spectra which were not detected during the MCD check.
Based on these the next step was to impose Fuzzy Structure Generation with the options shown in Figure 5. Normally Fuzzy Generation will only take action if there are no structures generated during strict structure generation, but by forcing it to start with extending one bond overcomes this.
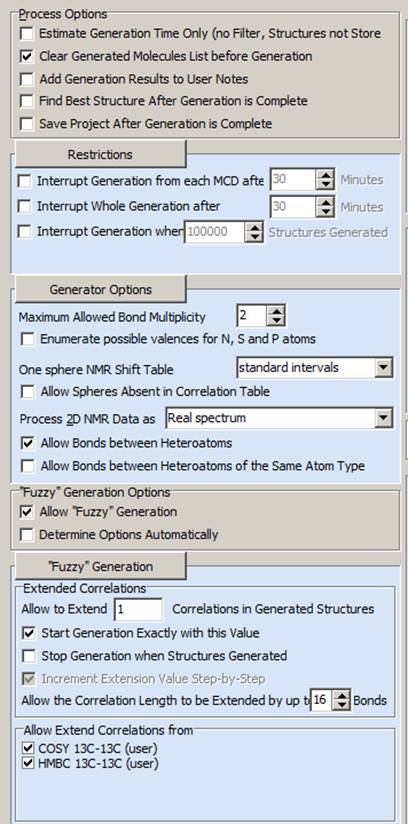
Figure 5. Options of Fuzzy Structure Generation.
Figure 5 shows that we suggested that one NSC of unknown length is present in the combined COSY and HMBC data. The results of Fuzzy Structure Generation are: k = 39353→(Filtering)→98→ (Removing duplicates) →94, tg = 44 s.
The 94 generated structures were then ranked in ascending order of average deviations between experimental and predicted by HOSE codes chemical shifts, dA(13C). The top six structures after the ranking are shown in Figure 6.
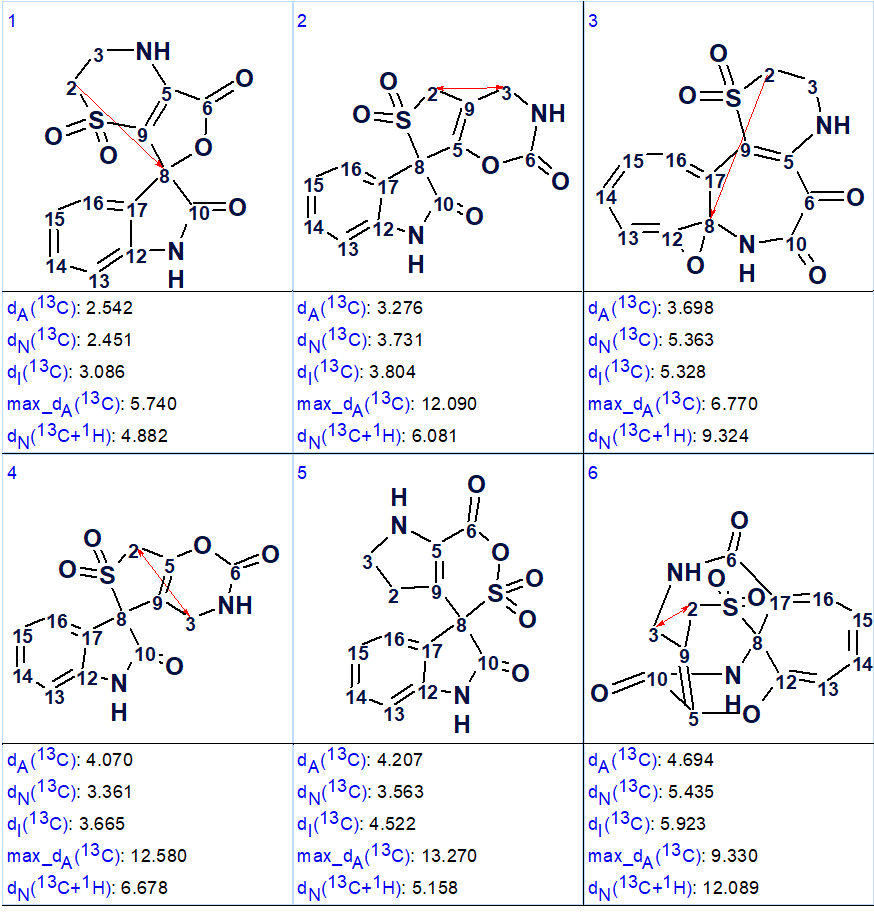
Figure 6. Six top ranked structures of the output file produced by Fuzzy Structure Generation. Red arrows denote a non-standard 2D NMR connectivity (one-side directed arrow corresponds to NSC existing in HMBC, two-side arrow in COSY).
We see that the first ranked structure coincides with structure 1 suggested for orbicularisine by the authors of [1]. A four-bond HMBC correlation between H2/C8 was revealed in the elucidated structure. Moreover we see that the deviations between the experimental and the predicted chemical shift values are now much smaller.
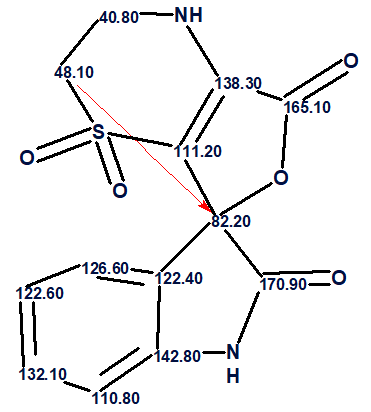
Thus, the correct structure of an unknown was determined using Fuzzy Structure Generation. The structure of orbicularisine together with the automatically assigned 13C chemical shifts is shown below:
References
- F. Goudou, P. Petit, C. Moriou, O. Gros, A. Al-Mourabit. (2017). “Orbicularisine: A Spiro-Indolothiazine Isolated from Gills of the Tropical Bivalve Codakia orbicularis“, J. Nat. Prod., 80: 1693−1696.


