January 1, 2015
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
Schiglautone A
Several plants from the genus Schisandra (of the family Schisandraceae) are widely used in Traditional Chinese Medicine. In addition to the presence of a large number of lignans, Schisandra was also found to be rich in triterpenoids with numerous pharmaceutical effects, which has aroused a lot of interest from pharmacologists. Schisandra glaucescens Diels is a vine plant mainly distributed in China, whose stems have been used for the treatment of various diseases in traditional medicine. The chemical constituents and pharmacological potential of S. glaucescens have never been reported. Meng et al [1] investigated potentially biologically active substances from the chemical constituents of the stems of S. glaucescens. As a result a novel triterpenoid possessing an unusual 6/7/9-fused tricyclic ring system was obtained, which was designated as Schiglautone A (1).
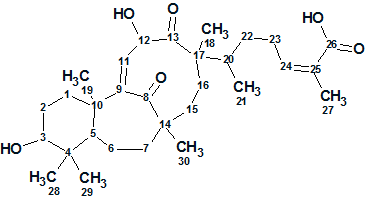
1
Schiglautone A was obtained as a colorless crystal with a molecular formula of C30H46O6 as deduced from HRESIMS data (m/z 501.3204 [M + H]+, calculated for 501.3216), requiring eight degrees of unsaturation. The 1H NMR data of structure 1 indicated the existence of seven methyls, two olefinic methines, and two oxymethines. The spectroscopic NMR data (1D NMR, HSQC, HMBC and COSY) are presented in Table 1.
Table 1: The spectroscopic NMR data of Schiglautone A.
| Label | δC | δC calc | CHn | δH | M(J) | COSY | C HMBC |
| C 1 | 29.2 | 34.08 | CH2 | 2.36 | u | 1.77 | C 9, C 3, C 10, C 19 |
| C 1 | 29.2 | 34.08 | CH2 | 1.43 | u | C 19, C 3, C 9 | |
| C 2 | 26 | 27.15 | CH2 | 1.95 | u | ||
| C 2 | 26 | 27.15 | CH2 | 1.77 | u | 2.36, 3.57 | C 1, C 3 |
| C 3 | 74.2 | 78.02 | CH | 3.57 | u | 1.77 | C 5 |
| C 4 | 40.1 | 39.52 | C | ||||
| C 5 | 41.8 | 54.23 | CH | 2.43 | u | 1.6 | C 7, C 19, C 9, C 3 |
| C 6 | 23.1 | 22.12 | CH2 | 1.38 | u | ||
| C 6 | 23.1 | 22.12 | CH2 | 1.6 | u | 2.43, 1.51 | C 14 |
| C 7 | 39.3 | 41.43 | CH2 | 1.21 | u | ||
| C 7 | 39.3 | 41.43 | CH2 | 1.51 | u | 1.6 | |
| C 8 | 214.7 | 205.96 | C | ||||
| C 9 | 152.3 | 150.75 | C | ||||
| C 10 | 38.3 | 41.18 | C | ||||
| C 11 | 123.1 | 138.27 | CH | 5.74 | u | 5.27 | C 8, C 9, C 10, C 12 |
| C 12 | 70.5 | 73.79 | CH | 5.27 | u | 5.74 | C 17, C 13, C 11, C 9 |
| C 13 | 215.9 | 217.2 | C | ||||
| C 14 | 50.2 | 47.35 | C | ||||
| C 15 | 34.3 | 36.57 | CH2 | 2.54 | u | 2.21 | C 17, C 30, C 16, C 8 |
| C 15 | 34.3 | 36.57 | CH2 | 1.232 | u | C 8, C 17 | |
| C 16 | 31.9 | 31.83 | CH2 | 2.21 | u | 2.54 | C 15, C 14, C 18, C 17 |
| C 16 | 31.9 | 31.83 | CH2 | 1.64 | u | C 17, C 13 | |
| C 17 | 57.2 | 51.73 | C | ||||
| C 18 | 16.3 | 18.34 | CH3 | 1.32 | u | C 13, C 22, C 17, C 20 | |
| C 19 | 20.7 | 25 | CH3 | 1.2 | u | C 5, C 9, C 10 | |
| C 20 | 41.6 | 33.53 | CH | 1.7 | u | 1.02, 1.27 | C 17, C 21, C 13 |
| C 21 | 13.7 | 15.86 | CH3 | 1.02 | u | 1.7 | C 22, C 20, C 17 |
| C 22 | 31.6 | 31.06 | CH2 | 1.89 | u | C 17, C 21, C 23, C 24 | |
| C 23 | 28.8 | 29.87 | CH2 | 2.75 | u | 1.27, 5.98 | C 22, C 25, C 20, C 24 |
| C 23 | 28.8 | 29.87 | CH2 | 2.9 | u | C 20, C 22, C 25, C 24 | |
| C 24 | 142.4 | 144.08 | CH | 5.98 | u | 2.75 | C 26, C 27, C 22 |
| C 25 | 128.4 | 127.57 | C | ||||
| C 26 | 170.4 | 172.34 | C | ||||
| C 27 | 21.2 | 20.75 | CH3 | 2.05 | u | C 26, C 24, C 25 | |
| C 28 | 21.8 | 27.14 | CH3 | 0.77 | u | C 29, C 4, C 3 | |
| C 29 | 29.2 | 16.15 | CH3 | 1.16 | u | C 28, C 4, C 3 | |
| C 30 | 25.8 | 22.3 | CH3 | 1.5 | u | C 7, C 8, C 14, C 15 |
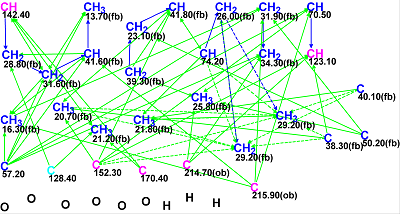
Figure 1: The Molecular Connectivity Diagram for Schiglautone A. No MCD edits were made.
MCD overview. As the degree of unsaturation (8) is not large, the number of HMBC and COSY connectivities is sufficient to allow us to omit the stage of MCD editing. Multiplicities determined in the 1H NMR spectrum were also not used.
MCD checking revealed the presence of at least one nonstandard connectivity in the 2D NMR data. Fuzzy Structure Generation was initiated in the mode “Determine Options Automatically” which was completed with the following results: k=2→2→2, tg = 0.015 s, with 1 of 59 correlations having been extended during generation, and 1 from 59 possible connectivity combinations used. Both of the generated structures are shown in Figure 2.
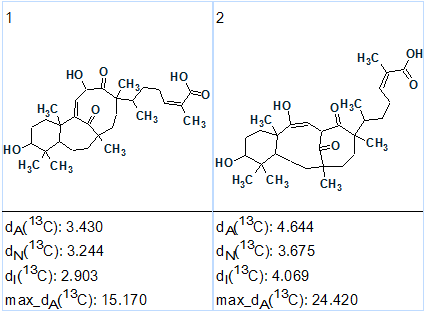
Figure 2: The output structures as ranked by 13C average deviations for Schiglautone A.
The first ranked structure is identical to structure 1. The structure of Schiglautone A supplied with 13C chemical shift assignment (2) is shown below. The red arrow denotes a single nonstandard connectivity.
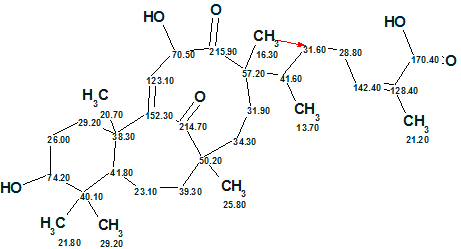
2
A structure containing 36 skeletal atoms and a unique 6/7/9-fused skeleton was therefore elucidated almost instantly (tg = 0.015 s!) and unambiguously in the fully automated mode of Fuzzy Structure Generation. This was possible owing to the rich HMBC and COSY data and successful automatic setting of the carbon atom properties. In this case, the initial set of axioms can be characterized as “complete” but contradictory.
References
- Meng FY, Sun JX, Li X, Yu HY, Li SM, Ruan HL. (2011). Schiglautone A, a new tricyclic triterpenoid with a unique 6/7/9-fused skeleton from the stems of Schisandra glaucescens. Org Lett 13(6):1502–1505. doi:10.1021/ol200188n


