April 1, 2020
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
Spiroaxillarone A
A phytochemical investigation of the aerial parts of C. axillaris led Wisetsai at al [1] to the isolation of a new compound belonging to the spirobisnaphthalene family with an unprecedented skeleton, spiroaxillarone A (1).
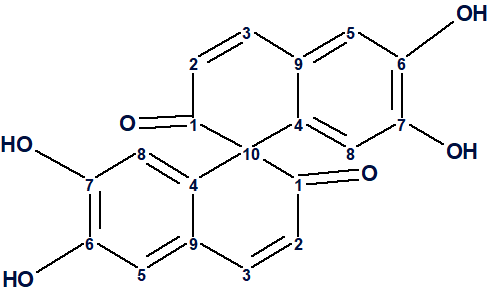
1
Spirobisnaphthalenes are a relatively new and rare family of secondary metabolites. Their core structure usually consists of two bicyclo[4.4.0]decane units, connected with one another through at least one spiro center. The reported compound is a novel symmetric spirobisnaphthalene with a unique structural feature. Spiroaxillarone A (1) possesses two monomeric naphthalene units connected through a spiro center (2H,2′H-1,1′-spirobi- [naphthalene]-2,2′-dione core). It is the first carbocyclic representative in the spirobisnaphthalene family, i.e., the first member not containing an oxygenated spiro heterocycle linking the two naphthalene subunits. The carbon skeleton of 1 is unprecedented in any naturally occurring compounds. [1]
Spiroaxillarone A (1) was isolated as a brown amorphous solid. UV absorption bands at λmax 265 and 370 nm indicated the presence of aromaticity. The IR spectrum showed the presence of hydroxy and α,β-unsaturated carbonyl groups at 3300 and 1635 cm−1, respectively. There were no bands in the 2100-2300 cm-1 region which indicates the absence of sp carbons (triple bonds). The 1H NMR spectrum of 1 looked quite simple, as only four signals appeared in the aromatic region with similar integration. The 13C NMR spectrum, however, showed 10 carbon signals including an unexpected, weak peak in the aliphatic region. The authors [1] suspected the presence of two monomeric naphthalene units connected in a symmetric fashion through a spiro carbon center. The molecular formula was determined as C19H12O6 by high resolution mass spectrometry. The ratio of skeletal atoms to hydrogens is equal to 2, which classifies this molecule as a hydrogen deficient one. The fact that the molecular formula has 19 carbon atoms but only 10 signals are observed confirms the fact that this is a symmetric molecule, and every carbon signal should correspond to two carbon atoms except one, which should correspond to the carbon atom at the link point. From the intensities of the peaks observed in the carbon spectrum it is clear that this carbon atom must be the one at 68.8 ppm, as it has at most half the intensity of the other carbons, including the one at 198.7 which is probably a carbonyl and known to appear weak.
The spectroscopic data used for structure elucidation of spiroaxillarone A were utilized for challenging the ACD/Structure Elucidator (ACD/Structure Elucidator). The data are summarized in Table 1.
Table 1. NMR spectroscopic data of compound 1
| Label | δC | δC calc* | CHn | δH | M(J) | COSY | H to C HMBC |
| C 1 | 198.700 | 196.390 | C | ||||
| C 2 | 120.600 | 121.950 | CH | 5.920 | d(9.6) | 7.50 | C 10, C 9 |
| C 3 | 149.500 | 147.940 | CH | 7.500 | d(10.0) | 5.92 | C 5, C 4, C 1 |
| C 4 | 136.300 | 129.140 | C | ||||
| C 5 | 116.800 | 115.500 | CH | 6.830 | s | C 4, C 6, C 7, C 3 | |
| C 6 | 144.700 | 146.550 | C | ||||
| C 7 | 148.500 | 146.330 | C | ||||
| C 8 | 115.900 | 115.370 | CH | 6.210 | s | C 10, C 9, C 6, C 7 | |
| C 9 | 122.000 | 123.910 | C | ||||
| C 10 | 68.800 | 63.400 | C |
13C NMR chemical shifts were predicted by the HOSE code method implemented into ACD/Structure Elucidator.
The molecular connectivity diagram (MCD) created from the data presented in Table 1 is shown in Figure 1.
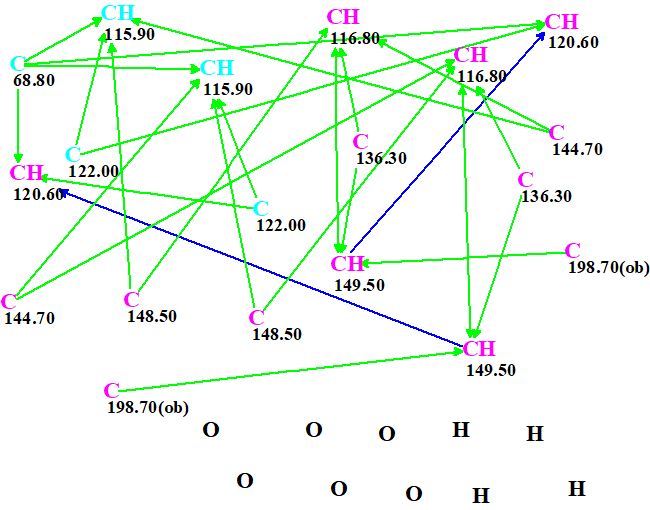
Figure 1. Molecular connectivity diagram.
The MCD contains five carbon atoms whose hybridization is ambiguous, that is sp2 or sp3 (not sp). These atoms are distinguished by light blue color. The hybridization of the remaining carbons is set as sp2 (violet) by the program. Atoms C 198.7 are marked by symbol ob, which means that these atoms must be connected to heteroatoms – to oxygens in this case. The presence of pairs of carbons with identical chemical shift in the MCD is a feature of a symmetric molecule.
In spite of the facts that structure elucidation of symmetric molecules from 2D NMR spectra is challenging for CASE systems and the molecular formula is hydrogen deficient, no edits of the MCD were made and structure generation was initiated. Result: k = 992 → 2 → 1, tg= 3 m 13 s.
The single structure generated by the program is shown in Figure 2.
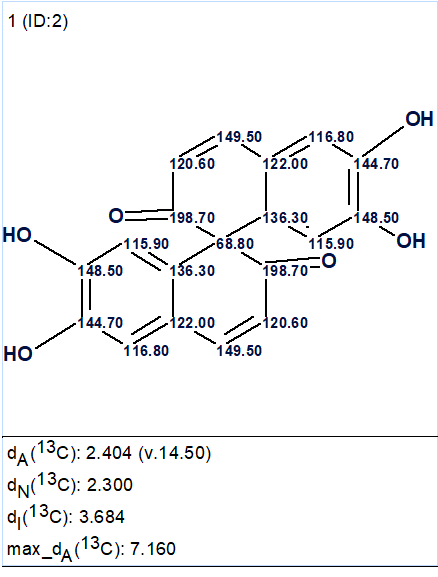
Figure 2. The single structure generated by the program. 13C chemical shift assignment was performed automatically.
We see that the structure is identical to that deduced by the authors [1] and confirmed by X-ray crystallography. Therefore, this unprecedented symmetric structure characterized by hydrogen deficiency was automatically elucidated by ACD/Structure Elucidator in a pretty reasonable time.
References
- A. Wisetsai, R. Lekphrom, J. Boonmak, S.Youngme, F.T. Schevenels. (2019). Spiroaxillarone A, a Symmetric Spirobisnaphthalene with an Original Skeleton from Cyanotis axillaris. Org. Lett. 21: 8344–8348.


