April 1, 2014
by Mikhail Elyashberg, Leading Researcher, ACD/Labs
TAEMC161 (Viridol)
Sakuno et al [1] isolated an aflatoxin biosynthesis enzyme inhibitor with molecular formula C20H18O6, which was labeled as TAEMC161. The following structure for this alkaloid was suggested from the 1D NMR, HMBC and NOE data:
1
During the process of structure elucidation the authors [1] postulated that the 13C chemical shift at 173.50 ppm was associated with the resonance of the ester group carbon. The authors wrote: “One ketone and one ester group were indicated by carbon resonances at d 206.7 and d 173.4 and IR bands at 1671 and 1707 cm-1.”
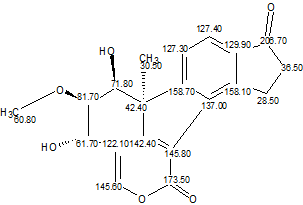
The original spectral data were input into ACD/Structure Elucidator Suite, and the Molecular Connectivity Diagram (MCD, Figure 1) was created.
Figure 1. Molecular Connectivity Diagram. Ketone and ester groups were drawn by hand.
Similar to Sakuno et al, the O=C—O group was involved in the process of fuzzy structure generation by manually adding it to the Molecular Connectivity Diagram. The results were: k=174→80→60, tg = 8 s. Three top structures of the ordered output file are shown in figure 2.
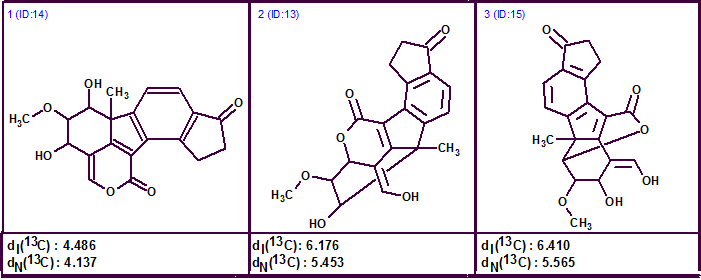
Figure 2. Three top structures of the ranked output file.
Figure 2 shows that structure 1 occupied the first ranked position, but with deviation values of about 4.5 ppm. Such large deviations suggest caution and close inspection of the data. It should be remembered that the typical accuracy of chemical shift calculation is about 1.6-1.8 ppm, placing this result outside of the normal range of acceptable accuracy.
Wipf and Kerekes [2] compared the NMR and IR spectra of TAEMC161 with spectra of a number of structural relatives and found close similarity between the spectra of TAEMC161 and viridol, 2:
2
In this molecule the carbonyl groups at 173.5 and 206.7 ppm are both ketones, and the structure is in accordance with the 2D NMR data used for deducing structure 1. Density functional theory calculations of the 13C chemical shifts were performed by Wipf and Kerekes [2] for structures 1 and 2 using GIAO approximation. It was proven that TAEMC161 is actually identical to 2.
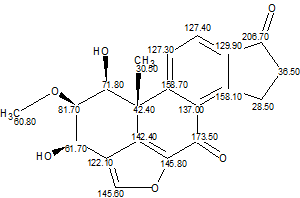
We again created the Molecular Connectivity Diagram (Figure 3) and repeated structure generation without any constraints imposed on the carbonyl groups. The following results were obtained: k=494→398→272, tg=40 s. The three top structures in the ranked output file are presented in Figure 4.
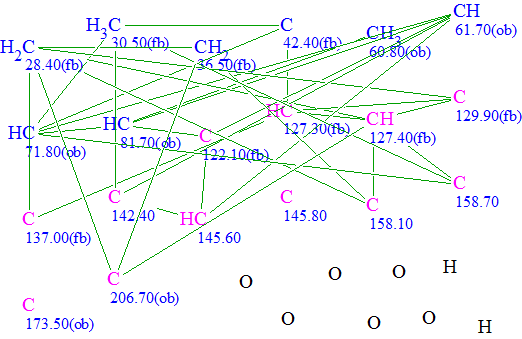
Figure 3. Molecular Connectivity Diagram “as is” (no user intervention).
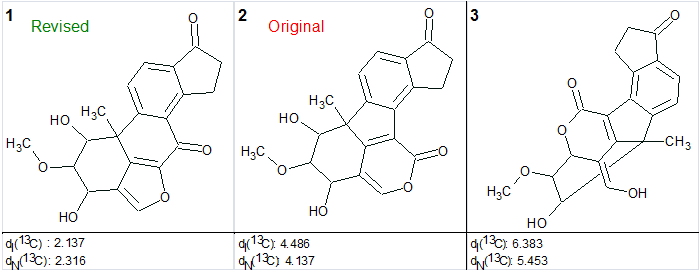
Figure 4. The top three structures of the output file generated for compound C20H18O6 (viridol).
The figure shows that empirical prediction of 13C chemical shifts convincingly demonstrates the superiority of the revised structure over the original suggested for TAEMC161.
The example clearly shows how an improper axiom assumption by a researcher can lead to an incorrect structure. Employing an unbiased analysis of spectral data using Structure Elucidator Suite would allow the authors [1] to infer the right structure unambiguously in several seconds. Many more examples of Structure Elucidator Suite application for the structure revision can be found in our 2010 review [3].
References
- E. Sakuno, K. Yabe, T. Hamasaki, H. Nakajima. A New Inhibitor of 5′-Hydroxyaverantin Dehydrogenase, an Enzyme Involved in Aflatoxin Biosynthesis, from Trichoderma hamatum” J. Nat. Prod. 2000, 63, 1677-1678.
- P. Wipf, A.D. Kerekes. Structure Reassignment of the Fungal Metabolite TAEMC161 as the Phytotoxin Viridiol. J. Nat. Prod. 2003, 66, 716-718.
- M. Elyashberg, A. Williams, K. Blinov. Structural revisions of natural products by Computer Assisted Structure Elucidation (CASE) Systems. Nat. Prod. Rep., 2010, 27(9):1296-1328.


