February 23, 2023
by Sanji Bhal, Director, Marketing & Communications, ACD/Labs
Analytical experiments are carried out to answer, essentially, one question: What is in my sample?
There is nuance based on the work being done—lead optimization in discovery, impurity control in process development, forced degradation studies, or manufacturing QA/QC. Results may be used to better understand a material, to understand a process, or to reveal its limitations—but the high-level question is still, “what is it?” The primary role of NMR, LC/MS, FTIR, Raman spectroscopy, and a variety of spectroscopic and analytical techniques is to verify or elucidate the identity of chemical (or biochemical) structures and mixture components.
For decades, ACD/Labs has been committed to supporting scientific experts from many of the world’s leading biopharmaceutical and chemical companies in identifying, verifying, and elucidating chemical structures. We offer a variety of products to help scientists accelerate this work (learn about our structure elucidation and structure verification product and solution offerings here). Recently, we were able to bring together some of these experts to discuss their workflows and how they’ve improved efficiency and confidence in structure determination, in a Structure Elucidation & Verification Virtual Symposium.
Meet Scientists at the Forefront of Structure Elucidation & Verification

As part of the chemical structure investigation team at Novartis, Dorina and her team are responsible for structure elucidation and impurity investigation for new chemical entities in development and commercialized products. They also provide proof of structure data for regulatory submissions, carrying out 400 elucidations and 700 structure verifications a year.
Dorina Kotoni, Associate Director/Chemical Structure Investigation Group Lead, Novartis
 Head of the Structural Analysis team at Sanofi, Celine and her team support synthetic chemistry research at 2 sites. They undertake >10,000 analyses a year in support of intellectual property and patent applications. While they primarily work on small molecules, recent years have seen an increase in antibody drug conjugates.
Head of the Structural Analysis team at Sanofi, Celine and her team support synthetic chemistry research at 2 sites. They undertake >10,000 analyses a year in support of intellectual property and patent applications. While they primarily work on small molecules, recent years have seen an increase in antibody drug conjugates.
Celine Nguyen-Van-Dau, Head of Integrated Drug Discovery (IDD) Structural Analysis team, Sanofi
 John is in the Structure Elucidation team at Pfizer. Their Analytical Research and Development department is split across the Atlantic Ocean in the UK and the US. His team works on samples in Phase II and Phase III clinical trials, which involves a lot of impurity identification.
John is in the Structure Elucidation team at Pfizer. Their Analytical Research and Development department is split across the Atlantic Ocean in the UK and the US. His team works on samples in Phase II and Phase III clinical trials, which involves a lot of impurity identification.
John Baker, Principal Scientist, Pfizer
 Cindy and Joe are responsible for identification and quantitation of all components in extractables and leachables (E&L) studies for medical devices at Medtronic. The purchase of a new instrument led them to re-think their process for E&L characterization and led to the implementation of new software and more robust processes.
Cindy and Joe are responsible for identification and quantitation of all components in extractables and leachables (E&L) studies for medical devices at Medtronic. The purchase of a new instrument led them to re-think their process for E&L characterization and led to the implementation of new software and more robust processes.
Cindy Roberson, Principal Scientist, Medtronic; Joe Buchman, Senior Chemist, Medtronic
 An analytical chemist in a team covering the breadth of global R&D at AstraZeneca, Kevin and his colleagues work with chemists, formulators, toxicologists, safety reps, and individuals from operations. AstraZeneca are focused on leveraging existing knowledge to speed analytical work and feed AI/ML projects in the future.
An analytical chemist in a team covering the breadth of global R&D at AstraZeneca, Kevin and his colleagues work with chemists, formulators, toxicologists, safety reps, and individuals from operations. AstraZeneca are focused on leveraging existing knowledge to speed analytical work and feed AI/ML projects in the future.
Kevin Robbins, Associate Principle Scientist, AstraZeneca
 Purnima, is involved in structure determination of macro-cyclic peptidomimetic compounds at Bristol Myers Squibb (BMS). Her team helps with determination of structure-activity-relationships (SAR), protein binding, and provides full structures to research colleagues.
Purnima, is involved in structure determination of macro-cyclic peptidomimetic compounds at Bristol Myers Squibb (BMS). Her team helps with determination of structure-activity-relationships (SAR), protein binding, and provides full structures to research colleagues.
Purnima Khandelwal, Senior Principal Scientist, Small Molecule Drug Discovery, Bristol Myers Squibb
These experts were the speakers at the symposium. Here are some of the over-arching discussion topics raised:
What Analytical Techniques Should You Use for Structure Determination?
The real answer is it depends on how much structural information you have—is it a novel compound requiring elucidation or are you verifying the presence of an expected structure?
What technique to use also depends on the type of compounds you anticipate are present—small molecule or biologic? Volatile or polar? Are there distinctive structural features that lend it to quick identification with a particular technique, e.g., Infra-red or UV?
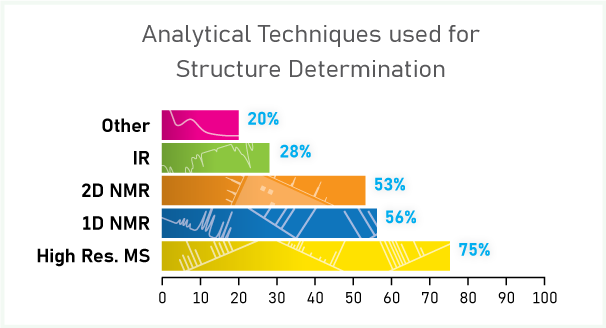
LC/MS and NMR were the primary techniques used by all of our experts. NMR is more popular when there is less certainty of the structure, for solution-phase structural information on peptides, or to provide greater certainty in structure characterization. The hierarchy of experiments was 1D 1H and/or 13C, followed by more time-consuming 2D NMR experiments. High resolution mass spectrometry (or accurate mass) is the go-to technique when a proposed structure is available.
As the only presenter talking about biological compounds, Purnima uses NMR to collect solution phase structure information on compounds at physiological pH. Beyond this, BMS scientists also use x-ray crystallography for 3D structural conformation.
We asked the audience (approximately 400 scientists from discovery and development) what techniques they use for structure determination. Three quarters use high resolution mass spectral data, almost equal numbers (56% and 53%) use 1D and 2D NMR data, just over one quarter (28%) use infrared spectroscopy, and 20% use a variety of other techniques.
Automated Structure Verification or Manual?
While this is the question many ask, it need not be so black and white. Companies are integrating different levels of automation depending on their needs. Automation is also becoming more attractive due to an ever-growing emphasis on efficiency and the shrinking headcount of analytical groups.
The Structure Elucidation team at Pfizer verify structures manually but they use NMR Workbook Suite for the tedious work of NMR data processing and analysis.
“I’m old enough to remember the days of assigning spectra with a pen and paper and I would not go back there.”
John Baker, Pfizer
When asked if they used automation for data processing and structure assignment, only 30% of the audience said they automate some or all of their structure verification work. But the majority are making plans for automated data analysis.
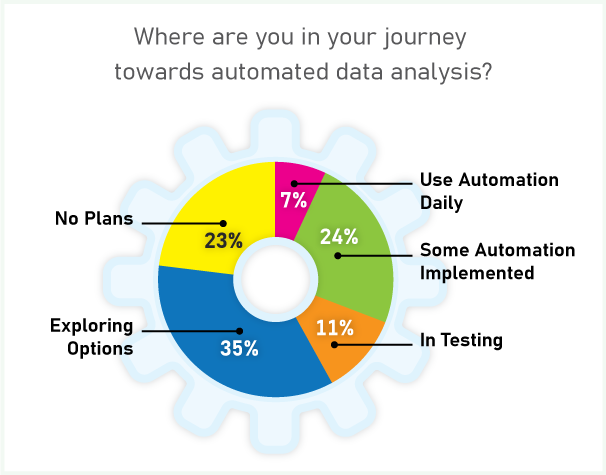
Sanofi first implemented automated structure verification by NMR in 2012. Their goals were to:
- Reduce the burden of routine structure verification on analysts.
- Create more time for elucidation of unknowns and complex structures.
- Speed up NMR reporting for patents and publications.
A decade later, they have undertaken an expansion to their Open Access lab that will also include LC/MS data. Their objective is for results to be delivered directly to the requesting chemist by email.
“The combined LC/MS and NMR verification system manages data from 2 UHPLC/MS systems, with 2 different LC methods each, and 3 different 400MHz NMR spectrometers. It delivers results as a PDF report for automated purity, agreement of 1H NMR spectrum with structure, and a multiplet report, with links to the live data for review/correction.”
Celine Nguyen-Van-Dau, Sanofi
Novartis have implemented automated structure verification by NMR, for molecules smaller than 800 Daltons across research and development. Analysts review the generated results which are then reported directly to the chemist, to a database, or to an ELN record, based on their needs.
“Our major focus is on structure elucidation so we want to work as little as possible on [analyses] where we feel that we know already what the structure is, or where we have data from previous analyses of the same structure.”
Dorina Kotoni, Novartis
Time Savings from Automated Structure Verification (ASV)
Dorina reported that her team at Novartis has saved a significant amount of time from their “ASV engine” deployment. She estimates that for manual verification an experienced analyst, on average, would spend 20 minutes on processing and interpreting NMR data per submission. When the ASV engine successfully delivers a structure confirmation result, the analyst only spends a minute or two on review, which represents a 90% time savings. When minor manual corrections are necessary, the analyst may spend 5 minutes on the analysis—less than half the time required for manual analysis. Even when ASV does not generate a result or data processing is incomplete, it still saves the analyst time “because the peak picking is done”, Kotoni adds.
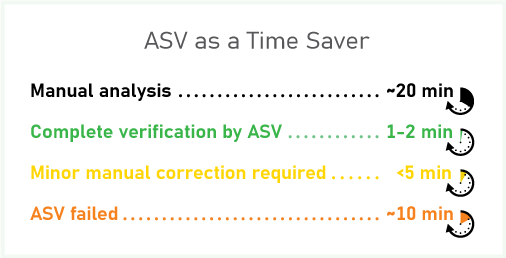
“After 2 years we are seeing 60–65% of successful automated verifications and the software is learning as we feed more data into it… Even failed verifications save the analysts time because they don’t have to process the datasets from scratch.”
Dorina Kotoni, Novartis
Benefits of Databasing in Structure Elucidation and Verification
Knowledge management has been a driver for the digitalization of analytical information over the last decade. All our presenters talked about databasing analytical and chemical information to some degree for a variety of purposes. These were the most compelling cases:
Improved Spectral Prediction for Automated Structure Verification Workflows
For those that have automated structure verification, the benefits of databasing spectral data are immediate and real. No commercial database will contain the novel structures being investigated in your organization. To improve the accuracy of NMR prediction for novel chemical space it is crucial to add your own curated data. Our experts not only agree, they talked about the results they have seen from this undertaking.
“Accurate prediction is very important for our automated structure verification process and we’ve seen excellent results from training the NMR predictors DB with or own data over the years.”
Celine Nguyen-van-dau, Sanofi
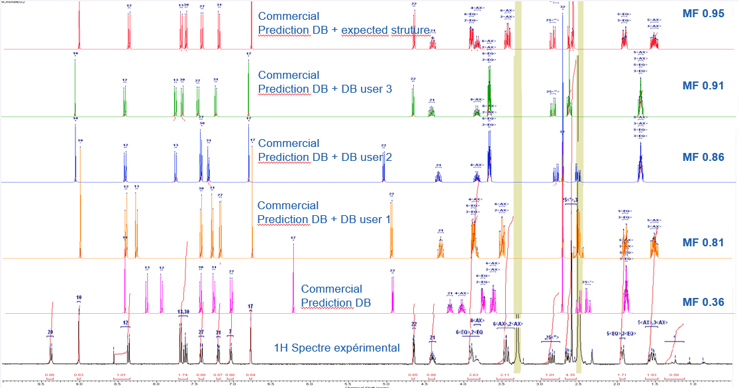
Celine shared an example of Sanofi’s results from training the NMR prediction database. They saw the match factor (comparing experimental to predicted spectrum) improve from 0.36 using the commercial database only, to 0.91 with the inclusion of 3 similar structures.
Confidence in Structure Elucidation and Structure Identification
While Pfizer scientists have not fully automated their structure verification workflow, they compare major chemical shifts with predicted carbon spectra to see correlations and provide confidence in their identifications.
“[Comparing experimental and predicted data] is a nice unbiased check to make sure that we’re not making unfounded assumptions about our structure, or we’ve not made some sort of human error,” says John.
The Structure Elucidation team have automated the inclusion of structure and spectral data to their C and H prediction training databases. John concludes that it only takes a few similar structures to improve prediction accuracy.
“Over the course of a 2-year project the root mean square error for 13C NMR predictions went from 2.5pmm to 0.2ppm.”
John Baker, Pfizer
Cindy and Joe, who rely heavily on spectral matching, retention time prediction, and fragment prediction to identify components in their extractables and leachables samples, also agree.
“We add data for identified structures into our database to increase the confidence in future elucidations.”
Cindy Roberson, Medtronic
Share Data and Collaborate More Effectively
Dorina and Kevin talked quite extensively about managing data in a central location.
“Since we generate and handle so much data, we need to make sure that it’s findable, usable, and in a central and easily accessible location”, says Kevin.
In response, the team at AstraZeneca have created the Global Analytical Database (GAD) to store metadata (instrument/method parameters), sample ID, structure, and all related analytical data (NMR, MS, and Chromatography). The GAD is a central, searchable database for AZ analysts across the world.
“[our Global Analytical Database] saves everyone a lot of time in writing reports, patents, and publications. You no longer have to contact the analyst and wait days to retrieve data. It’s all in a single platform that you can search to find what you need.”
Kevin Robbins, AstraZeneca
At Novartis, the chemical structure investigation team has a view to manage all their development data, including forced degradation and stability studies, in Luminata®. Multiple departments are involved in the development work—formulations, analytical, and chemistry. Sharing data between these groups has historically been challenging. Dorina and her colleagues’ aim is for Luminata to facilitate data exchange between teams and to provide a better understanding and overview of their processes.
“We have all the information about a chemical entity (impurity or any other type of compound) in one place with Luminata. The synthetic route in which it was observed and where, its MW, the stage and batch, reference analytical data, HPLC of the particular batch and the NMR spectrum of that specific compound.” Dorina Kotoni, Novartis.
Analysts are forever pushing the boundaries of structure determination. Keeping an eye on instrument innovations that let them learn more from ever-smaller quantities of sample. Using all the information they can glean from orthogonal techniques and past elucidations to make confident decisions, faster. A big thank you to our presenters. Access their presentations here, and let us know about other insights you extract, or reach out with any questions.



I extracted compound from a plant. I would like to carry out structural elucidation and interpretation using NMR, IR, 13 C. Can your lab. help me? Secondly what does it take and how can I send the sample?
Hi Israel, we can suggest software to support your structure elucidation efforts, we are not a lab that analyses samples